Hidradenitis suppurativa (HS) is a chronic inflammatory disease manifesting in inverse body regions. In HS, there appears to be a dysregulated adipokine release that is shifted towards pro-inflammatory adipokines. Insulin resistance is significantly more common in HS than in healthy patients regardless of BMI, age, and gender. Insulin resistance in HS patients leads to further cardiovascular disease. The mechanism of insulin resistance and role of adipokines should be investigated in future studies to better provide the pathomechanisms of HS. The role of androgens seems to be important in a certain subgroup of female patients. Anti-androgenic therapy can be useful and helpful in some patients.
- hidradenitis suppurativa
- acne inversa
- hormones
- spironolactone
- metformin
- finasteride
- insulin resistance
- thyroid function
- endocrinology
- adipokine
1. Introduction
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic, inflammatory skin disease of inverse body regions. HS is a globally underestimated disease with a prevalence of 0.4–1% [1][2]. Some data describe a prevalence of 4% [3]. The disease is accompanied by inflammatory nodules, abscesses, scars, contractures, and fistulas that significantly reduce the patient’s quality of life. Therapy of HS consists of local antiseptics, local antibiotics, systemic antibiotics, high-dose zinc therapy, biologics, and surgical interventions [4]. In advanced disease with many fistulas, surgery and combined immunomodulatory therapies can be indicated. In some cases, preoperative downstaging with antibiotics may be useful. There is currently no curative therapy as the pathogenesis is not yet fully understood. According to current knowledge, the pathogenesis of HS is multifactorial. HS is associated with smoking, obesity, and metabolic syndrome. Use of hormonal drugs for treatment of HS is discussed in the literature. In some cases, taking medications such as spironolactone, which also have an anti-hormonal effect, leads to an improvement in HS [5][6].
The hormonal role in the pathogenesis of HS is not yet fully understood. A worsening of HS is often described by patients premenstrually and after pregnancy [7]. Therefore, a hormonal change could be a cause of HS. Many patients with HS have impaired glucose tolerance and insulin resistance. There are some case control studies describing an improvement in HS under antidiabetic or antiandrogenic therapy.
2. HS Manifestation and Exacerbation
The hormonal influence on HS is evident in the typical fluctuations of the disease. HS usually begins peripubertally and shows exacerbation after pregnancy. Detailed analysis of the timing from the fluctuations could provide further insight into HS pathogenesis. Perimenstrually, 43–76.7% of patients report subjective worsening in HS [7][11]. A meta-analysis has shown that about 25% of the patients report improvement and 20% of the patients report worsening in HS during pregnancy [12]. However, about 60% of the patients show worsening in HS postpartum [12]. All these results support the hormonal influence in HS.
3. Sexual Hormones in HS
It has long been suspected that pathophysiology of HS is linked to sex hormones. Sex hormones are steroid hormones that are produced in the gonads, placenta, or adrenal cortex. Hormones are especially important for human reproduction and formation of the sex. The release is controlled by the hypothalamic-–pituitary axis. The male sex hormones include androgens and female sex hormones include estrogens, gestagens, and hCG. The connection between HS and sex hormones is obvious, but, so far, the exact connection could be not clarified. HS shows clinical deterioration or improvement at certain times, characterized by hormonal fluctuations. During pregnancy, clinical improvement may occur in HS. The frequent peripubertal onset of HS and perimenstrual flares suggest that sex hormones are involved in HS pathogenesis.
In an experimental study, immunohistochemical expression of the androgen receptor (AR) and estrogen receptor (ER) in HS skin tunnels has been investigated [13]. HS showed increased AR expression in the infundibulum and skin tunnel compared to healthy skin. AR expression was higher in males than in females. Immunohistochemical ER expression was predominantly negative. In a microarray analysis of gene expression, HS lesions showed increased androgen receptor (AR) transcriptional activity compared to non-lesional skin [14]]. Apocrine glands in HS show no difference in AR and ER receptor expression [15]. Zouboulis’ study also showed a subordinate role of the apocrine gland in HS [16]. Interestingly, androgen-controlled genes were up-regulated in women, while genes affecting fat metabolism were down-regulated in men [16]. Mortimer et al. discovered early on that female HS patients have an elevated testosterone and free androgen index [17]]. There are three cases reported where cross-sex hormone therapy (CSHT) with testosterone resulted in an exacerbation or manifestation of HS [18][19]. In contrast to the above results, Harrison reports that there is no difference in testosterone between HS and healthy controls [20]. After stimulation by thyrotropin-releasing hormone and gonadotropin-releasing hormone, increased values were found for prolactin and TSH [20]. This is remarkable as usually the response to TRH testing is blunted in males compared to women [21][22]. Harrison et al. concluded that there might rather be a disturbance in the feedback signals of the peripheral hormones in HS. There are no recent data on the hypothalamic–pituitary axis.
4 Anti-Androgen Therapy in HS
The efficacy of different drugs with an anti-androgenic effect further supports the role of hormones in HS. Clinical improvement has been observed in female HS patients with the use of antiandrogenic drugs.
In a retrospective study by Kraft et al., 64 female patients with HS were included. Antiandrogenic therapy was more effective compared to antibiotic therapy (55% vs. 26%; p < 0.04) [23]. These positive results were included in the 2019 North American guideline for HS. They recommend use of hormonal agents in appropriate patients and mild to moderate HS [24]. Estrogen-containing oral contraceptives, spironolactone, cyproterone acetate, metformin, finasteride, and flutamide should be used in combination with other agents [24].
There are some case series that show a positive effect of finasteride in HS [25][26][27][28]. Finasteride is a competitive inhibitor of steroid 5α-reductase and has an anti-androgenic effect. Conversion of testosterone into dihydrotestosterone (DHT) is selectively inhibited by finasteride. DHT is the biologically active form of testosterone. Testosterone circulates in blood and enters the cell through lipophilic character [29]. Within the cell, it is converted into DHT. DHT and the intracellular androgen receptor form a hormone–receptor complex that migrates into the cell nucleus and binds to a hormone-responsive element (HRE) [29]. This interaction thus influences gene expression. In Germany and the USA, finasteride is approved in a daily dose of 1 mg for androgenic alopecia and 5 mg for benign prostatic hyperplasia [30]. In all four reports, the patients showed a significant improvement in HS [25][26][27][28]. Under therapy with finasteride, there was a decrease in frequency and intensity of HS relapses. In some patients, use of finasteride resulted in complete healing of lesions. In most cases, a daily dose of 5 mg was used and well-tolerated by the patients. In rare cases, headache, nausea, menstrual irregularities, breast tenderness, or decreased libido/sexual function have been described as adverse events [27]. It should be noted that all case series reported predominantly about female patients, so adverse events could be significantly more severe in male patients [31]. Overall, finasteride appears to be a safe and effective treatment option for HS.
In recent years, use of spironolactone in young female patients has increased significantly [32]. In the age group of 13 to 19 years, its usage has increased three times compared to previous years [32][33]. Spironolactone is a competitive aldosterone receptor antagonist and has weak antiandrogenic, estrogenic, and glucocorticoid effects. Spironolactone is a potassium-sparing diuretic and is licensed for treatment of severe heart failure, resistant hypertension, nephrotic syndrome, liver cirrhosis, and Conn’s syndrome [34]. The response to spironolactone in HS ranges from 42 to 85% [5][35]. There was no difference between monotherapy with spironolactone and combination with other drugs [36]. Spironolactone can be administered in a dose of 25 to 200 mg [5][6][35][37]. A daily dose of 100 mg has been administered most frequently. All reports of antiandrogenic therapy with spironolactone in HS are from female patients. Spironolactone also has a positive effect on quality of life [37]. A randomized phase 4 trial was designed to investigate the efficacy and optimal dose of spironolactone in HS (ClinicalTrials.gov Identifier: NCT04100083). The study was discontinued due to lack of funding.
One of the first hormonal drugs used in HS is cyproterone acetate (CPA). CPA is a competitive antagonist at the androgen receptor and agonist at the progesterone receptor. CPA prevents testosterone and dihydrotestosterone from binding to the receptor. CPA also lowers serum levels of gonadotropins LH and FSH. Further, 21-hydroxylase is also inhibited by CPA so that synthesis of mineral corticoids (e.g., aldosterone) or glucocorticoids (e.g., cortisol) is inhibited. There are positive reports regarding use of CPA in HS [23][38][39]. In a randomized trial, CPA has been compared with norgestrel [38]. There was no significant difference between the two groups. Both drugs showed a similar response.
Flutamide has a non-steroidal anti-androgenic effect. One case report reports an improvement in HS under flutamide with a dose of 250 mg daily [40]. The antidiabetic drug metformin has a mild anti-androgenic effect and is used in PCOS.
5. Insulin Resistance and Adipokines
An association between HS and metabolic syndrome has been reported in several studies [41]. Truncal obesity, hypertension, diabetes mellitus type II, and dyslipoproteinemia, characteristics of metabolic syndrome, are frequently found in HS patients. Metabolic syndrome is present in 32.4% of HS patients [42]. A recent meta-analysis estimated the pool ratio of metabolic syndrome in HS to be 2.66 (95% CI: 1.90-3.72) [43]. There is also an association between HS and diabetes mellitus [44][45]. A meta-analysis showed that there is a 1.69-fold increased risk of developing diabetes mellitus in HS [46]. A cross-sectional analysis in the United States showed an overall prevalence of 24.8% for type 2 diabetes mellitus in HS [47]. In the same cohort, the prevalence of type 2 diabetes mellitus in non-HS patients was 15.6%. In a comparative cross-sectional study of 3207 patients, a significant association was presented between HS and metabolic syndrome [odds ratio (OR) 1.61, 95% confidence interval (CI) 1.36-1.89], diabetes mellitus (OR 1.41, 95% CI 1.19-1.66), obesity (OR 1.71, 95% CI 1.53-1.91), hyperlipidemia (OR 1.14, 95% CI 1.02-1.28), and hypertension (OR 1.19, 95% CI 1.03-1.38) [48]. Many patients have impaired glucose tolerance. The exact mechanism of impaired tolerance is not clear.
There is some research on adipokine levels in HS. Adipokines are signaling molecules produced by adipose tissue. They act as a link between the immune system and energy metabolism. With weight gain, the pro-inflammatory effect increases, and, with hunger, the anti-inflammatory effect increases. Known adipokines include plasminogen activator inhibitor-1 (PAI-1), leptin, visfatin, adiponectin, apelin, interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1), retinol binding protein 4 (RBP4), TNF-α, omentin-1, and vaspin. Secretion and expression of adipokines is disturbed in cardiovascular diseases and obesity [49]. In a case-control study by Malara et al., the adipokines adiponectin, resistin, and leptin in blood were compared to healthy controls, nondiabetic obese group, and psoriasis patients [50]. The mean serum levels of adiponectin were significantly decreased in patients with HS compared to healthy lean controls, and the resistin and leptin levels were increased [50]. In all the patient groups, BMI and serum levels of leptin (r=0.83) and resistin (r=0.6) were strongly correlated. The authors suggested that serum levels of adipokines are dysregulated in HS and are associated with obesity [50]. Expression of adipokines in this study was shifted towards pro-inflammatory resistin and leptin. Akdogan et al. found that serum levels of visfatin after adjustment for BMI and smoking status differed significantly (P = 0.02) and increased the risk of HS 1.56-fold [51]. González-López also reports that visfatin and resistin are independent risk factors for HS [52]. Both adipokines were independent of age, sex, and body mass index. Adiponectin was inversely associated with IR (OR 0.994; CI 95%, 0.989-0.999; p = 0.023) and resistin positively (OR 1.012; CI 95%, 1.001-1.024; p = 0.03) after adjustment (age, sex, BMI, and smoking status) [52]. However, there seems to be no correlation between serum levels of adipokines and severity of HS [52]. Another adipokine is retinol binding protein 4 (RBP4), which is produced by fat cells [53]. RBP4 is elevated in insulin resistance and also in diabetes mellitus [54][55]. RBP4 is also a transport protein for free vitamin A [56]. In a cross-sectional study of 137 patients (77 HS patients and 60 controls) without diabetes mellitus, higher RBP4 and lower ghrelin levels were found in HS [57]. RBP4 levels correlated positively with disease severity and insulin resistance in HS patients independent of BMI [57]. There was no correlation between disease severity and ghrelin. Omentin-1 was also found to be elevated in HS patients compared to healthy controls after adjustment for BMI, age, and sex [58]. Omentin-1 is an adipocytokine expressed in visceral fat. Omentin-1 has an important role in body metabolism and insulin sensitivity via AMP-activated protein kinase [59].
Increased incidence of insulin resistance in HS also suggests a disturbed hormonal axis [42][51][60]. Insulin resistance has often been determined by the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR). HOMA-IR is calculated from the product of fasting insulin (µu/ml) and fasting blood glucose (mg/dl), which is then divided by 405 [61]. A HOMA-IR value above 2.5 may indicate insulin resistance. In a cross-sectional and case-control study, 76 patients with HS and 61 age- and sex-matched control subjects have been compared for the presence of insulin resistance [60]. The median HOMA-IR value was significantly higher in HS than in controls (2.0 vs. 1.5; p = 0.01) [60]. Prevalence of IR was also significantly higher than in controls (43.4% vs. 16.4%; p = 0.001) [60]. The authors, therefore, recommend that HS patients should be screened for IR. The study by Akdogan et al. and Özkur et al. also showed a significant correlation between HS and insulin serum levels [42,51]. Weight loss in HS patients has a positive effect on insulin resistance and can lead to a decrease in HS lesions and insulin resistance [62]. This supports the central role of insulin resistance in HS (Figure 1).
Metformin is an oral antidiabetic drug that increases the effect of insulin. The insulin sensitizer has the effect of reducing glucose production in liver and increasing glucose utilization by muscle and fat cells. Metformin also has an anti-inflammatory effect on several cell types. It has been reported by Chung et al. that metformin reduces production of nitric oxide (NO), prostaglandin E2 (PGE2), and pro-inflammatory cytokines, such as IL-6, IL-1β and TNF-α [63]. This is probably reduced by inhibiting activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in macrophages. A therapeutic response of metformin in HS patients has already been described in the literature (Table 7). In a prospective study (n=25) with metformin, 72% of the patients experienced clinical improvement in HS [63]. DLQI also improved in 64% of the patients. In a retrospective study of children with HS, response to therapy was 50% of the patients [64]. In Jennings’ study, most patients on metformin therapy have been analyzed so far [65]. The study showed a treatment response of 68 %. In 19% of the patients, use of metformin led to complete remission. It was interesting to note that 75% of the patients had insulin resistance [65]. Overall, metformin has been well-tolerated by patients. The most common side effect was gastrointestinal symptoms [63][64][65]. The effect of metformin on HS is currently being investigated in the first double-blind randomized trial called “Rediscovery of Metformin in Chronic Invalidating Autoinflammatory Disease Hidradenitis Suppurativa” (ClinicalTrials.gov Identifier: NCT04649502). The primary endpoint of the metformin study is clinical response, assessed by IHS4. Secondary endpoints include insulin resistance, lesion count, pain, HS-PGA, cost-effectiveness, change in biomarker calprotectin, relapses, treatment satisfaction, DLQI, safety, and tolerability. In the first study arm, patients receive metformin and doxycycline. In the placebo arm, patients receive placebo and doxycycline. The first results of the metformin trial are expected in 2023.
Another antidiabetic drug is liraglutide. There is only one case report of clinical experience with liraglutide in HS [66][67]. Liraglutide is a GLP-1 agonist and directly stimulates GLP-1 receptor. Stimulation of GLP-1 results in glucose-dependent insulin secretion and inhibition of glucagon secretion. As with metformin, most common side effects of liraglutide include gastrointestinal symptoms. In rare cases, pancreatitis and pancreatic carcinoma can occur. After four weeks of liraglutide therapy, an obese patient who was not insulin-resistant experienced clinical improvement in HS and DLQI [66]. HS-PGA decreased from 4 to 1 and DLQI from 24 to 14 under liraglutide.
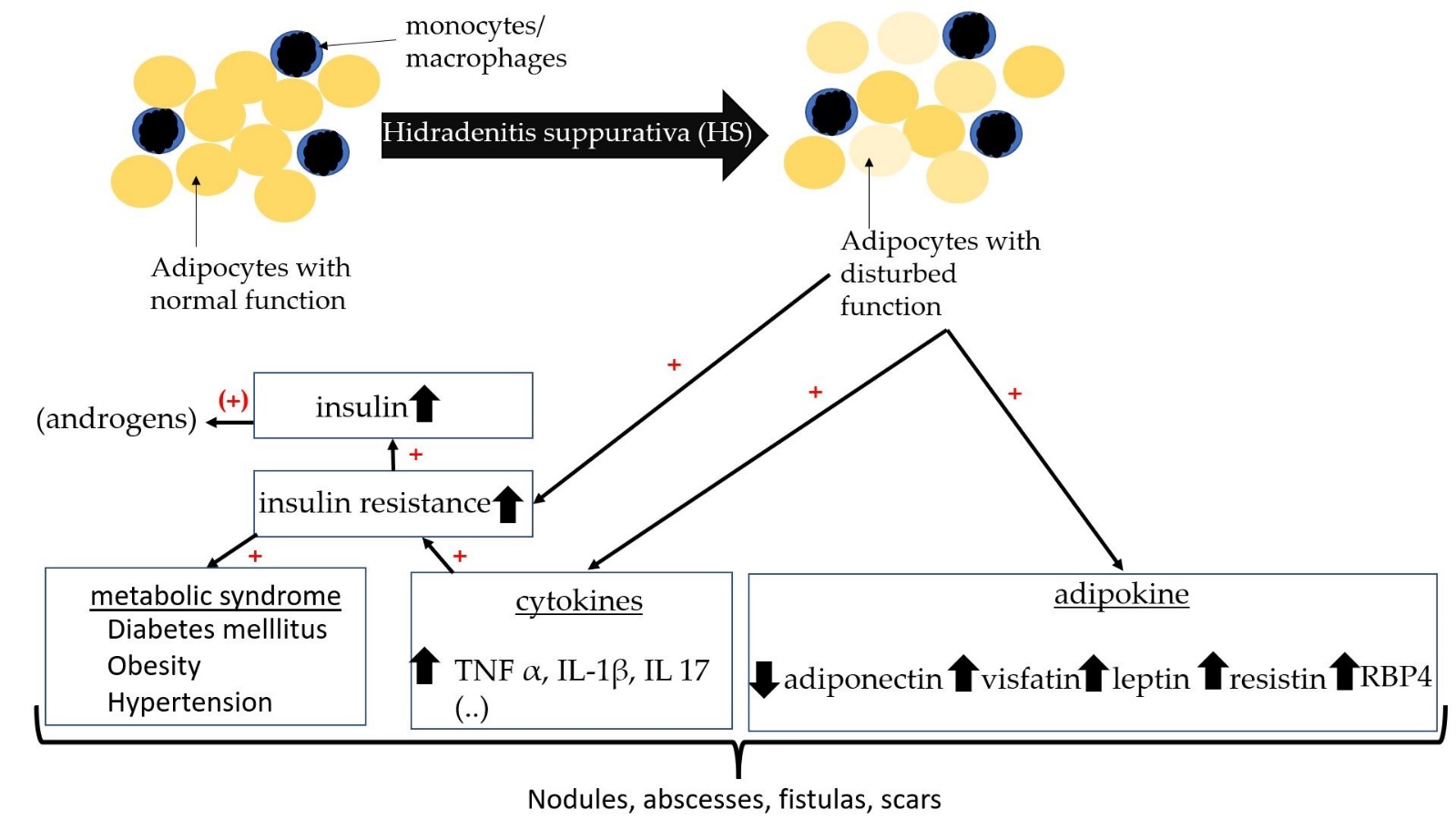
Figure 1. Schematic diagram with relationship between insulin resistance, adipokines, and HS.
6. Thyroid Function in HS
The thyroid gland also plays an important role in human hormone balance. In the thyroid gland, iodine-containing hormones including triiodothyronine (T3) and thyroxine (T4) are produced by thyrocytes. Both hormones are important in metabolic process and growth. T3 is biologically more active than T4 and is produced from T4 by type 1 and 2 deiodinases. Thyroid hormones act on many organs. In general, two hormones have an increasing effect on energy metabolism. Thyroid hormones affect the heart by increasing expression of β-receptors and heart rate [68]. In metabolism, they increase basal metabolic rate and oxygen consumption. Glucose absorption, gluconeogenesis, and glycogen synthesis are increased. Regulation of the thyroid gland takes place via the hypothalamus–pituitary axis. Thyroid-stimulating hormone (TSH) from the pituitary gland is released after stimulation by thyrotropin-releasing hormone (TRH) from the hypothalamus. TSH stimulates T3 and T4 synthesis. In hyperthyroidism, there is increased production of the hormones. Patients complain of tachycardia, weight loss, increased body temperature, tremor, sleep disturbances, and high nervousness. In hypothyroidism, patients have bradycardia, weight gain, decreased body temperature, fatigue, and dry skin. In addition, the hormone calcitonin is produced in the C-cells of the thyroid gland. Calcitonin is mainly involved in calcium balance and lowers short-term calcium in the blood.
Large case-control studies and cohort studies have shown that thyroid disease is a common comorbidity of HS [44][69]. Liakou et al. described that severity of HS was significantly correlated with presence of thyroid disease and active smoking [70]. In a population-based cross-sectional study of 4191 people, the association between HS and thyroid disease has been investigated [71]. There was an increased odds ratio for hypothyroidism (OR 2.91; 95% CI: 2.48-3.40; p<0.001) and hyperthyroidism (OR 2.25; 95% CI: 1.55-3.28; p<0.001) in HS compared to controls. The association between HS and hypothyroidism was also confirmed via multivariate logistic regression analysis. Even after controlling for age, sex, socioeconomic status, and smoking, the association remained significant. The association between HS and hyperthyroidism was not significant in the adjusted model, so HS was only independently significant with hypothyroidism. In contrast to the results of Sherman et al., the study by Miller et al. showed a significant correlation between HS and increased TSH and decreased free T3 [36][71]. Miller et al. retrospectively examined blood of 430 HS patients for thyroid hormones. Clinical hyperthyroidism was also significantly associated after removal of confounding factors (OR of 1.91; 95% CI 1.19-3.07; p=0.02) [36]. HS with vulvar involvement (VHS) is also associated with thyroid disease. López-Llunell et al. studied 25 patients with VHS and found that patients had late onset of disease. The average BMI of VHS was normal (HS with VHS: 23.2 kg/m2 vs. HS without: VHS 28.6 kg/m2) [72]. A nationwide cohort analysis in Denmark investigated medication of HS patients [73]. No increased hazard ratio for thyroid medication could be found. The authors assume that comorbidities such as thyroid disease occur in severe courses [73]. Gonzoalez-Lopez et al. also reported that thyroid function parameters did not differ between HS patients and controls [74]. Autoimmune antibodies against the thyroid gland also do not differ significantly between HS and non-HS patients [45][74].
7. Polycystic Ovary Syndrome (PCOS) and HS
Another comorbidity of HS in women is also polycystic ovary syndrome (PCOS). PCOS is an endocrine disorder characterized by hyperandrogenism, cycle disorder, and, less commonly, polycystic ovaries. Many patients demonstrate a phenotype of metabolic syndrome. Pathophysiologically, insulin resistance is present, leading to increased androgen synthesis by IGF-1 in the ovary. Hyperandrogenemia leads to virilization and LH dominance. Patients with PCOS may receive anti-androgenic therapy with metformin or oral contraceptives. The prevalence of PCOS in HS is 9% according to available data. In a meta-analysis with five case-control studies, HS patients were shown to have a 2.64-fold greater risk of PCOS [75]. Screening for PCOS can be useful in certain patients[76][77][78][79][80][81][82][83][84][85][86][87].
This entry is adapted from the peer-reviewed paper 10.3390/ijms232315250
References
- Jfri, A.; Nassim, D.; O’Brien, E.; Gulliver, W.; Nikolakis, G.; Zouboulis, C.C. Prevalence of Hidradenitis Suppurativa: A Systematic Review and Meta-regression Analysis. JAMA Dermatol. 2021, 157, 924–931. https://doi.org/10.1001/jamadermatol.2021.1677.
- Ingram, J.R.; Collins, H.; Atkinson, M.D.; Brooks, C.J. The prevalence of hidradenitis suppurativa is shown by the Secure Anonymised Information Linkage (SAIL) Databank to be one per cent of the population of Wales. Br. J. Dermatol. 2020, 183, 950–952. https://doi.org/10.1111/bjd.19210.
- Jemec, G.B.; Heidenheim, M.; Nielsen, N.H. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J. Am. Acad. Dermatol. 1996, 35, 191–194. https://doi.org/10.1016/S0190-9622(96)90321-7.
- Zouboulis, C.C.; Bechara, F.G.; Fritz, K.; Kurzen, H.; Liakou, A.I.; Marsch, W.C.; Milling, A.; Nast, A.; Podda, M.; Taube, K.M.; et al. S1-Leitlinie zur Therapie der Hidradenitis suppurativa/Acne inversa* (ICD-10 Ziffer: L73.2). J. Dtsch. Dermatol. Ges. 2012, 10 (Suppl. 5), S1–S31. https://doi.org/10.1111/j.1610-0387.2012.08006.x.
- Lee, A.; Fischer, G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas. J. Dermatol. 2015, 56, 192–196. https://doi.org/10.1111/ajd.12362.
- Golbari, N.M.; Porter, M.L.; Kimball, A.B. Antiandrogen therapy with spironolactone for the treatment of hidradenitis suppurativa. J. Am. Acad. Dermatol. 2019, 80, 114–119. https://doi.org/10.1016/j.jaad.2018.06.063.
- Fernandez, J.M.; Hendricks, A.J.; Thompson, A.M.; Mata, E.M.; Collier, E.K.; Grogan, T.R.; Shi, V.Y.; Hsiao, J.L. Menses, pregnancy, delivery, and menopause in hidradenitis suppurativa: A patient survey. Int. J. Women’s Dermatol. 2020, 6, 368–371. https://doi.org/10.1016/j.ijwd.2020.07.002.
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264-9, W64. https://doi.org/10.7326/0003-4819-151-4-200908180-00135.
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. https://doi.org/10.1111/j.1365-2230.1994.tb01167.x.
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 2017, 177, 1401–1409. https://doi.org/10.1111/bjd.15748.
- Vossen, A.R.J.V.; van Straalen, K.R.; Prens, E.P.; van der Zee, H.H. Menses and pregnancy affect symptoms in hidradenitis suppurativa: A cross-sectional study. J. Am. Acad. Dermatol. 2017, 76, 155–156. https://doi.org/10.1016/j.jaad.2016.07.024.
- Seivright, J.R.; Villa, N.M.; Grogan, T.; Parvataneni, R.K.; Thompson, A.M.; Shi, V.Y.; Hsiao, J.L. Impact of Pregnancy on Hidradenitis Suppurativa Disease Course: A Systematic Review and Meta-Analysis. Dermatology 2022, 238, 260–266. https://doi.org/10.1159/000517283.
- Yu, W.; Barrett, J.; Liu, P.; Parameswaran, A.; Chiu, E.S.; Lu, C.P. Novel evidence of androgen receptor immunoreactivity in skin tunnels of hidradenitis suppurativa: Assessment of sex and individual variability. Br J Dermatol 2021, 185, 855–858. https://doi.org/10.1111/bjd.20520.
- Gauntner, T.D. Hormonal, stem cell and Notch signalling as possible mechanisms of disease in hidradenitis suppurativa: A systems-level transcriptomic analysis. Br J Dermatol 2019, 180, 203–204. https://doi.org/10.1111/bjd.17093.
- Buimer, M.G.; Wobbes, T.; Klinkenbijl, J.H.G.; Reijnen, M.M.P.J.; Blokx, W.A.M. Immunohistochemical analysis of steroid hormone receptors in hidradenitis suppurativa. Am. J. Dermatopathol. 2015, 37, 129–132. https://doi.org/10.1097/DAD.0000000000000206.
- Zouboulis, C.C.; Da Nogueira Costa, A.; Fimmel, S.; Zouboulis, K.C. Apocrine glands are bystanders in hidradenitis suppurativa and their involvement is gender specific. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1555–1563. https://doi.org/10.1111/jdv.16264.
- Mortimer, P.S.; Dawber, R.P.; Gales, M.A.; Moore, R.A. Mediation of hidradenitis suppurativa by androgens. Br. Med. J. (Clin Res. Ed) 1986, 292, 245–248. https://doi.org/10.1136/bmj.292.6515.245.
- Buonomo, M.; Mansh, M.D.; Thorpe, D.; Goldfarb, N. Development or exacerbation of hidradenitis suppurativa in two transgender men after initiation of testosterone therapy. Br. J. Dermatol. 2021, 184, 1192–1194. https://doi.org/10.1111/bjd.19812.
- Ramos-Rodríguez, D.; Garcias-Ladaria, J.; Serra Soler, G.; Martín-Santiago, A. Hidradenitis suppurativa in a transgender man. Clin. Exp. Dermatol. 2021, 46, 1305–1306. https://doi.org/10.1111/ced.14680.
- Harrison, B.J.; Kumar, S.; Read, G.F.; Edwards, C.A.; Scanlon, M.F.; Hughes, L.E. Hidradenitis suppurativa: Evidence for an endocrine abnormality. Br. J. Surg. 1985, 72, 1002–1004. https://doi.org/10.1002/bjs.1800721223.
- Targum, S.D.; Marshall, L.E.; Magac-Harris, K.; Martin, D. TRH tests in a healthy elderly population. Demonstration of gender differences. J. Am. Geriatr. Soc. 1989, 37, 533–536. https://doi.org/10.1111/j.1532-5415.1989.tb05685.x.
- Dysken, M.W.; Falk, A.; Pew, B.; Kuskowski, M.; Krahn, D.D. Gender differences in TRH-stimulated TSH and prolactin in primary degenerative dementia and elderly controls. Biological Psychiatry 1990, 28, 144–150. https://doi.org/10.1016/0006-3223(90)90631-B.
- Kraft, J.N.; Searles, G.E. Hidradenitis suppurativa in 64 female patients: Retrospective study comparing oral antibiotics and antiandrogen therapy. J. Cutan. Med. Surg. 2007, 11, 125–131. https://doi.org/10.2310/7750.2007.00019.
- Alikhan, A.; Sayed, C.; Alavi, A.; Alhusayen, R.; Brassard, A.; Burkhart, C.; Crowell, K.; Eisen, D.B.; Gottlieb, A.B.; Hamzavi, I.; et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J. Am. Acad. Dermatol. 2019, 81, 91–101. https://doi.org/10.1016/j.jaad.2019.02.068.
- Joseph, M.A.; Jayaseelan, E.; Ganapathi, B.; Stephen, J. Hidradenitis suppurativa treated with finasteride. J. Dermatolog. Treat. 2005, 16, 75–78. https://doi.org/10.1080/09546630510031403.
- Mota, F.; Machado, S.; Selores, M. Hidradenitis Suppurativa in Children Treated with Finasteride-A Case Series. Pediatr. Dermatol. 2017, 34, 578–583. https://doi.org/10.1111/pde.13216.
- Babbush, K.M.; Andriano, T.M.; Cohen, S.R. Antiandrogen therapy in hidradenitis suppurativa: Finasteride for females. Clin. Exp. Dermatol. 2022, 47, 86–92. https://doi.org/10.1111/ced.14847.
- Randhawa, H.K.; Hamilton, J.; Pope, E. Finasteride for the treatment of hidradenitis suppurativa in children and adolescents. JAMA Dermatol. 2013, 149, 732–735. https://doi.org/10.1001/jamadermatol.2013.2874.
- Naamneh Elzenaty, R.; Du Toit, T.; Flück, C.E. Basics of androgen synthesis and action. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101665. https://doi.org/10.1016/j.beem.2022.101665.
- Salisbury, B.H.; Tadi, P. StatPearls: 5 Alpha Reductase Inhibitors; StatPearls Publishing, FL, USA, 2022.
- Abdelmaksoud, A. Comment on “Hidradenitis suppurativa in children treated with finasteride-A case series”. Pediatr. Dermatol. 2018, 35, 158. https://doi.org/10.1111/pde.13334.
- Horissian, M.; Maczuga, S.; Barbieri, J.S.; Zaenglein, A.L. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J. Am. Acad. Dermatol. 2022, 87, 684–686. https://doi.org/10.1016/j.jaad.2021.12.005.
- Barbieri, J.S.; James, W.D.; Margolis, D.J. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: A retrospective analysis, 2004-2013. J. Am. Acad. Dermatol. 2017, 77, 456-463.e4. https://doi.org/10.1016/j.jaad.2017.04.016.
- Patibandla, S.; Heaton, J.; Kyaw, H. StatPearls: Spironolactone; StatPearls Publishing, FL, USA, 2022.
- McPhie, M.L.; Bridgman, A.C.; Kirchhof, M.G. Combination Therapies for Hidradenitis Suppurativa: A Retrospective Chart Review of 31 Patients. J. Cutan. Med. Surg. 2019, 23, 270–276. https://doi.org/10.1177/1203475418823529.
- Miller, I.M.; Vinding, G.; Sørensen, H.A.; Rytgaard, H.; Mogensen, U.B.; Ellervik, C.; Jemec, G.B. Thyroid function in hidradenitis suppurativa: A population-based cross-sectional study from Denmark. Clin. Exp. Dermatol. 2018, 43, 899–905. https://doi.org/10.1111/ced.13606.
- Quinlan, C.; Kirby, B.; Hughes, R. Spironolactone therapy for hidradenitis suppurativa. Clin. Exp. Dermatol. 2020, 45, 464–465. https://doi.org/10.1111/ced.14119.
- Mortimer, P.S.; Dawber, R.P.; Gales, M.A.; Moore, R.A. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br. J. Dermatol. 1986, 115, 263–268. https://doi.org/10.1111/j.1365-2133.1986.tb05740.x.
- Sawers, R.S.; Randall, V.A.; Ebling, F.J. Control of hidradenitis suppurativa in women using combined antiandrogen (cyproterone acetate) and oestrogen therapy. Br J Dermatol 1986, 115, 269–274. https://doi.org/10.1111/j.1365-2133.1986.tb05741.x.
- Li, C.; Xu, H.; Zhang, X.; Zhang, W.; He, Y.; Wang, B. Hidradenitis suppurativa is treated with low-dose flutamide. J. Dermatol. 2019, 46, e52-e54. https://doi.org/10.1111/1346-8138.14541.
- Sabat, R.; Chanwangpong, A.; Schneider-Burrus, S.; Metternich, D.; Kokolakis, G.; Kurek, A.; Philipp, S.; Uribe, D.; Wolk, K.; Sterry, W. Increased prevalence of metabolic syndrome in patients with acne inversa. PLoS ONE 2012, 7, e31810. https://doi.org/10.1371/journal.pone.0031810.
- Özkur, E.; Erdem, Y.; Altunay, İ.K.; Demir, D.; Dolu, N.Ç.; Serin, E.; Çerman, A.A. Serum irisin level, insulin resistance, and lipid profiles in patients with hidradenitis suppurativa: A case-control study. An. Bras. Dermatol. 2020, 95, 708–713. https://doi.org/10.1016/j.abd.2020.04.009.
- Mintoff, D.; Benhadou, F.; Pace, N.P.; Frew, J.W. Metabolic syndrome and hidradenitis suppurativa: Epidemiological, molecular, and therapeutic aspects. Int. J. Dermatol. 2022, 61, 1175–1186. https://doi.org/10.1111/ijd.15910.
- Shlyankevich, J.; Chen, A.J.; Kim, G.E.; Kimball, A.B. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: A chart-verified case-control analysis. J. Am. Acad. Dermatol. 2014, 71, 1144–1150. https://doi.org/10.1016/j.jaad.2014.09.012.
- Lee, J.H.; Kwon, H.S.; Jung, H.M.; Kim, G.M.; Bae, J.M. Prevalence and comorbidities associated with hidradenitis suppurativa in Korea: A nationwide population-based study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1784–1790. https://doi.org/10.1111/jdv.15071.
- Phan, K.; Charlton, O.; Smith, S.D. Hidradenitis suppurativa and diabetes mellitus: Updated systematic review and adjusted meta-analysis. Clin. Exp. Dermatol. 2019, 44, e126-e132. https://doi.org/10.1111/ced.13922.
- Garg, A.; Birabaharan, M.; Strunk, A. Prevalence of type 2 diabetes mellitus among patients with hidradenitis suppurativa in the United States. J. Am. Acad. Dermatol. 2018, 79, 71–76. https://doi.org/10.1016/j.jaad.2018.01.014.
- Shalom, G.; Freud, T.; Harman-Boehm, I.; Polishchuk, I.; Cohen, A.D. Hidradenitis suppurativa and metabolic syndrome: A comparative cross-sectional study of 3207 patients. Br. J. Dermatol. 2015, 173, 464–470. https://doi.org/10.1111/bjd.13777.
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. https://doi.org/10.1016/j.jjcc.2013.11.006.
- Malara, A.; Hughes, R.; Jennings, L.; Sweeney, C.M.; Lynch, M.; Awdeh, F.; Timoney, I.; Tobin, A.M.; Lynam-Loane, K.; Tobin, L.; et al. Adipokines are dysregulated in patients with hidradenitis suppurativa. Br. J. Dermatol. 2018, 178, 792–793. https://doi.org/10.1111/bjd.15904.
- Akdogan, N.; Alli, N.; Uysal, P.I.; Topcuoglu, C.; Candar, T.; Turhan, T. Visfatin and insulin levels and cigarette smoking are independent risk factors for hidradenitis suppurativa: A case-control study. Archives of dermatological research 2018, 310, 785–793. https://doi.org/10.1007/s00403-018-1867-z.
- González-López, M.A.; Vilanova, I.; Ocejo-Viñals, G.; Arlegui, R.; Navarro, I.; Guiral, S.; Mata, C.; Pérez-Paredes, M.G.; Portilla, V.; Corrales, A.; et al. Circulating levels of adiponectin, leptin, resistin and visfatin in non-diabetics patients with hidradenitis suppurativa. Archives of dermatological research 2020, 312, 595–600. https://doi.org/10.1007/s00403-019-02018-4.
- Frances, L.; Tavernier, G.; Viguerie, N. Adipose-Derived Lipid-Binding Proteins: The Good, the Bad and the Metabolic Diseases. Int. J. Mol. Sci. 2021, 22, 460. https://doi.org/10.3390/ijms221910460.
- Lee, D.-C.; Lee, J.-W.; Im, J.-A. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism 2007, 56, 327–331. https://doi.org/10.1016/j.metabol.2006.10.011.
- Cho, Y.M.; Youn, B.-S.; Lee, H.; Lee, N.; Min, S.-S.; Kwak, S.H.; Lee, H.K.; Park, K.S. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 2006, 29, 2457–2461. https://doi.org/10.2337/dc06-0360.
- Kelly, M.; Widjaja-Adhi, M.A.K.; Palczewski, G.; Lintig, J. von. Transport of vitamin A across blood-tissue barriers is facilitated by STRA6. FASEB J. 2016, 30, 2985–2995. https://doi.org/10.1096/fj.201600446R.
- González-López, M.A.; Ocejo-Viñals, J.G.; Mata, C.; Vilanova, I.; Guiral, S.; Portilla, V.; Blanco, R.; Hernández, J.L. Association of retinol binding protein4 (RBP4) and ghrelin plasma levels with insulin resistance and disease severity in non-diabetic patients with hidradenitis suppurativa. Exp. Dermatol. 2020, 29, 828–832. https://doi.org/10.1111/exd.14132.
- González-López, M.A.; Ocejo-Viñals, J.G.; Mata, C.; Díaz, D.; Guiral, S.; Portilla, V.; Corrales, A.; González-Vela, M.C.; González-Gay, M.A.; Blanco, R.; et al. Evaluation of serum omentin-1 and apelin concentrations in patients with hidradenitis suppurativa. Postepy Dermatol. Alergol. 2021, 38, 450–454. https://doi.org/10.5114/ada.2021.107932.
- Watanabe, T.; Watanabe-Kominato, K.; Takahashi, Y.; Kojima, M.; Watanabe, R. Adipose Tissue-Derived Omentin-1 Function and Regulation. Compr. Physiol. 2017, 7, 765–781. https://doi.org/10.1002/cphy.c160043.
- Vilanova, I.; Hernández, J.L.; Mata, C.; Durán, C.; García-Unzueta, M.T.; Portilla, V.; Fuentevilla, P.; Corrales, A.; González-Vela, M.C.; González-Gay, M.A.; et al. Insulin resistance in hidradenitis suppurativa: A case-control study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 820–824. https://doi.org/10.1111/jdv.14894.
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. https://doi.org/10.1007/BF00280883.
- Macklis, P.C.; Tyler, K.; Kaffenberger, J.; Kwatra, S.; Kaffenberger, B.H. Lifestyle modifications associated with symptom improvement in hidradenitis suppurativa patients. Arch. Dermatol. Res. 2022, 314, 293–300. https://doi.org/10.1007/s00403-021-02233-y.
- Verdolini, R.; Clayton, N.; Smith, A.; Alwash, N.; Mannello, B. Metformin for the treatment of hidradenitis suppurativa: A little help along the way. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1101–1108. https://doi.org/10.1111/j.1468-3083.2012.04668.x.
- Moussa, C.; Wadowski, L.; Price, H.; Mirea, L.; O’Haver, J. Metformin as Adjunctive Therapy for Pediatric Patients With Hidradenitis Suppurativa. J. Drugs Dermatol. 2020, 19, 1231–1234. https://doi.org/10.36849/JDD.2020.5447.
- Jennings, L.; Hambly, R.; Hughes, R.; Moriarty, B.; Kirby, B. Metformin use in hidradenitis suppurativa. J. Dermatolog. Treat. 2020, 31, 261–263. https://doi.org/10.1080/09546634.2019.1592100.
- Jennings, L.; Nestor, L.; Molloy, O.; Hughes, R.; Moriarty, B.; Kirby, B. The treatment of hidradenitis suppurativa with the glucagon-like peptide-1 agonist liraglutide. Br. J. Dermatol. 2017, 177, 858–859. https://doi.org/10.1111/bjd.15233.
- Emtestam, L.; Sartorius, K. Glucagon-like peptide-1 agonists for treatment of hidradenitis suppurativa. Br. J. Dermatol. 2017, 177, 625–627. https://doi.org/10.1111/bjd.15742.
- Müller, P.; Leow, M.K.-S.; Dietrich, J.W. Minor perturbations of thyroid homeostasis and major cardiovascular endpoints-Physiological mechanisms and clinical evidence. Front. Cardiovasc. Med. 2022, 9, 942971. https://doi.org/10.3389/fcvm.2022.942971.
- Kimball, A.B.; Sundaram, M.; Gauthier, G.; Guérin, A.; Pivneva, I.; Singh, R.; Ganguli, A. The Comorbidity Burden of Hidradenitis Suppurativa in the United States: A Claims Data Analysis. Dermatol. Ther. (Heidelb) 2018, 8, 557–569. https://doi.org/10.1007/s13555-018-0264-z.
- Liakou, A.I.; Kontochristopoulos, G.; Marnelakis, I.; Tsantes, A.G.; Papadakis, M.; Alevizou, A.; Rotsiamis, N.; Rigopoulos, D. Thyroid Disease and Active Smoking May Be Associated with More Severe Hidradenitis Suppurativa: Data from a Prospective Cross Sectional Single-Center Study. Dermatology 2021, 237, 125–130. https://doi.org/10.1159/000508528.
- Sherman, S.; Tzur Bitan, D.; Kridin, K.; Pavlovsky, L.; Hodak, E.; Cohen, A.D. Hidradenitis suppurativa is associated with hypothyroidism and hyperthyroidism: A large-scale population-based study. Int. J. Dermatol. 2021, 60, 321–326. https://doi.org/10.1111/ijd.15319.
- López-Llunell, C.; Romaní, J.; Garbayo-Salmons, P.; Agut-Busquet, E. Vulvar hidradenitis suppurativa: Clinical cross-sectional study of 25 patients. J. Dermatol. 2021, 48, 457–463. https://doi.org/10.1111/1346-8138.15728.
- Andersen, R.K.; Loft, I.C.; Burgdorf, K.; Erikstrup, C.; Pedersen, O.B.; Jemec, G.B.E. Risk of Hidradenitis Suppurativa Comorbidities Over Time: A Prospective Cohort Study of Danish Blood Donors. Acta Derm. Venereol. 2021, 101, adv00376. https://doi.org/10.2340/00015555-3737.
- González-López, M.A.; Hernández, J.L.; Vilanova, I.; Mata, C.; López-Escobar, M.; González-Vela, M.C.; López-Hoyos, M.; González-Gay, M.A.; Blanco, R. Thyroid autoimmunity in patients with hidradenitis suppurativa: A case-control study. Clin. Exp. Dermatol. 2017, 42, 642–644. https://doi.org/10.1111/ced.13153.
- Phan, K.; Charlton, O.; Smith, S.D. Hidradenitis suppurativa and polycystic ovarian syndrome: Systematic review and meta-analysis. Australas. J. Dermatol. 2020, 61, e28-e33. https://doi.org/10.1111/ajd.13110.
- Garg, A.; Neuren, E.; Strunk, A. Hidradenitis Suppurativa Is Associated with Polycystic Ovary Syndrome: A Population-Based Analysis in the United States. J. Invest. Dermatol. 2018, 138, 1288–1292. https://doi.org/10.1016/j.jid.2018.01.009.
- Canoui-Poitrine, F.; Le Thuaut, A.; Revuz, J.E.; Viallette, C.; Gabison, G.; Poli, F.; Pouget, F.; Wolkenstein, P.; Bastuji-Garin, S. Identification of three hidradenitis suppurativa phenotypes: Latent class analysis of a cross-sectional study. J. Invest. Dermatol. 2013, 133, 1506–1511. https://doi.org/10.1038/jid.2012.472.
- Tzellos, T.; Zouboulis, C.C.; Gulliver, W.; Cohen, A.D.; Wolkenstein, P.; Jemec, G.B.E. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: A systematic review and meta-analysis of observational studies. Br J Dermatol 2015, 173, 1142–1155. https://doi.org/10.1111/bjd.14024.
- Gambichler, T.; Hessam, S.; Cramer, P.; Abu Rached, N.; Bechara, F.G. Complete blood collection-based systemic inflammation biomarkers for patients with hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1593–1596. https://doi.org/10.1111/jdv.18175.
- Sivanand, A.; Gulliver, W.P.; Josan, C.K.; Alhusayen, R.; Fleming, P.J. Weight Loss and Dietary Interventions for Hidradenitis Suppurativa: A Systematic Review. J. Cutan. Med. Surg. 2020, 24, 64–72. https://doi.org/10.1177/1203475419874412.
- Damiani, G.; Mahroum, N.; Pigatto, P.D.M.; Pacifico, A.; Malagoli, P.; Tiodorovic, D.; Conic, R.R.; Amital, H.; Bragazzi, N.L.; Watad, A.; et al. The Safety and Impact of a Model of Intermittent, Time-Restricted Circadian Fasting (“Ramadan Fasting”) on Hidradenitis Suppurativa: Insights from a Multicenter, Observational, Cross-Over, Pilot, Exploratory Study. Nutrients 2019, 11, 1781. https://doi.org/10.3390/nu11081781.
- Prasetya, G.; Sapwarobol, S. Intermittent Fasting During Ramadan Improves Insulin Sensitivity and Anthropometric Parameters in Healthy Young Muslim Men. Am. J. Lifestyle Med. 2021, 15, 200–206. https://doi.org/10.1177/1559827618815430.
- Gnanou, J.V.; Caszo, B.A.; Khalil, K.M.; Abdullah, S.L.; Knight, V.F.; Bidin, M.Z. Effects of Ramadan fasting on glucose homeostasis and adiponectin levels in healthy adult males. J. Diabetes Metab. Disord. 2015, 14, 55. https://doi.org/10.1186/s40200-015-0183-9.
- Gaeini, Z.; Mirmiran, P.; Bahadoran, Z. Effects of Ramadan intermittent fasting on leptin and adiponectin: A systematic review and meta-analysis. Hormones (Athens) 2021, 20, 237–246. https://doi.org/10.1007/s42000-021-00285-3.
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. https://doi.org/10.3390/ijms22147644.
- Abu Rached, Nessr; Gambichler, Thilo; Dietrich, Johannes W.; Ocker, Lennart; Seifert, Caroline; Stockfleth, Eggert; Bechara, Falk G.; The Role of Hormones in Hidradenitis Suppurativa: A Systematic Review. IJMS 2022, 23, 15250, https://doi.org/10.3390/ijms232315250.
- Chung, M.-M.; Nicol, C.J.; Cheng, Y.-C.; Lin, K.-H.; Chen, Y.-L.; Pei, D.; Lin, C.-H.; Shih, Y.-N.; Yen, C.-H.; Chen, S.-J.; et al. Metformin activation of AMPK suppresses AGE-induced inflammatory response in hNSCs. Exp. Cell Res. 2017, 352, 75–83. https://doi.org/10.1016/j.yexcr.2017.01.017.
