Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Medicinal
Heterocyclic compounds are cyclic compounds which contain the atoms of two discrete elements as representative of their ring(s). They belong to one of the larger classes of organic compounds and also appear more valuable in different fields of chemistry.
- heterocyclic compound
- medicinal chemistry
- infectious disease
1. Heterocyclic Compounds
Heterocyclic compounds are cyclic compounds which contain the atoms of two discrete elements as representative of their ring(s). They belong to one of the larger classes of organic compounds and also appear more valuable in different fields of chemistry [5]. These compounds encompass a higher number of drugs, most biomass, nucleic acids, synthetic dyes, and numerous natural products such as alkaloids, herbicides, vitamins, antibiotics, hormones, and pharmaceutics [6]. Heterocyclic compounds have tremendous importance for both organic and medical chemists, and the synthesis of such molecules always remains a challenge from both industrial and academic aspects [7]. Heterocyclic systems with different structures display various biologic activities because of diversities in their molecular structures [8]. All these systems have been used for the composition of unusual drugs due to their unique physicochemical properties. Furthermore, the systems of these compounds have been very attractive in recent times because of both sulfur and nitrogen atoms present in them [9]. In therapeutic perseverance, to deal with the various types of bacterial and fungal infections along with the analysis of cancer, gastric ulcers, and many other diseases, these compounds have been extensively utilized [10].
2. Antibacterial Activity of Heterocyclic Compounds
2.1. Synthesis and Antibacterial Activity of the Pyrrole
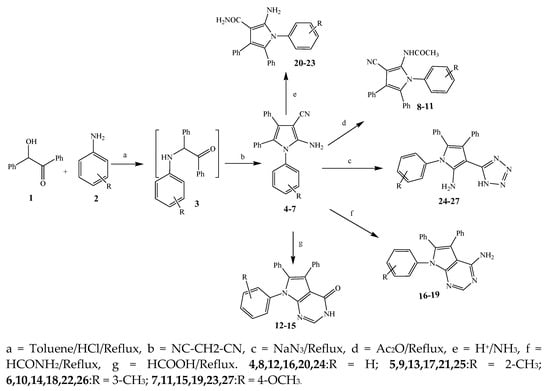
The synthesis of various derivatives of pyrrole (4–27) was carried out by Mohamed et al., 2009 from primary aromatic amines (2), benzoin (1), and malononitrile (Scheme 1). The synthesized compounds were evaluated in terms of their antibacterial activity against Staphylococcus aureus, Escherichia coli, Bacillus subtilis, and Pseudomonas aeruginosa. Most of the synthesized compounds exhibited excellent activity against these bacterial strains. Some of them showed similar activity while others were two to four times more or less active than the standard drug, amoxicillin. The activity of compounds 6, 10, and 15 was almost similar to that of the reference drug, whereas 17 was found to be two times more active than amoxicillin against B. subtilis. Furthermore, the compound 18 against S. aureus displayed activity that was lower than amoxicillin, while 21 and 27 were four times more active than amoxicillin. The compound 5 was also more effective against E. coli and 16 showed significant activity against P. aeruginosa, which was reciprocal to amoxicillin [16].
Scheme 1. Synthetic route of pyrrole and its derivatives (4–27).
Largani et al., 2017 also synthesized pyrrolo [3,4-b]quinolin-2(3H)-yl)benzamides from benzohydrazide and 9-phenylfuro[3,4-b]quinoline-1,3-dionesas as shown in Scheme 2. The antibacterial activity of compounds 37–41 was evaluated against Staphylococcus aureus, E. coli and Enterococcus faecalis which demonstrated vigorous inhibition against these bacterial strains. It was observed that amongst all the targeted compounds, 37 exhibited magnificent activity against E. faecalis, S. aureus and E. coli bacteria with MIC 0.5, 0.25, and 0.25 mg/mL, respectively [19]. The various pyrrole derivatives were prepared (Scheme 3 and Scheme 4) from amino-containing compounds through several steps and their antibacterial activity was evaluated [20]. Compound 42 was synthesized by refluxing acetophenone and thiourea, which was further used for the synthesis of targeted compounds. The synthesized compounds exhibited good antibacterial potential against microbes with an MIC value of 16 µg/mL (Table 1). The activity of compound 44 was equipotent correlated with ciprofloxacin against S. aureus and E. coli [21]. The protected pyrrole ring was synthesized (46–53) from 45 by reacting with aromatic aldehyde and protected amine. It was revealed that compounds 50, 51, and 52 exhibit valuable antibacterial activity against Pseudomonas putida bacterial strains. Compound 49 also showed the equivalent inhibitory potential against the tested strains of bacteria. Similarly, 53 and 49 exhibited remarkable antibacterial activity at the range of 32–64 μg/mL [20]. The synthesis of bis-pyrrole (56–60) through hydrazonoyl halides (Scheme 5) and their evaluation against four different bacterial strains was reported by Kheder [22]. The synthesized compounds were more active against the positive strains as compared to the negative strains. Furthermore, compound 59 with the Cl group at the para-position displayed a high degree of antibacterial activity against B. subtilis and S. aureus. Similarly, pyrrole derivatives (64–76) were prepared by a method based on microwave irradiation (Scheme 6) and their antibacterial activity was evaluated against E. coli and S. aureus [23]. It was also found that the compounds 65 and 71 exhibit significant activity against both S. aureus and E. coli, while 72 was the least effective. Furthermore, 71 displayed MIC (20 μg/mL in S. aureus and E. coli) closer to the reference drug, gentamicin. They also explained the SAR of the synthesized compounds, which showed that the substituent 3-formylpyrroles at position C-2 with thiophene (52) and pyridine (50, 51) had significant antibacterial activity, while 4-pyridyl had a weak response. They further elaborated that the presence of methoxy and ethoxy at positions C-8 and C-6 in the system of chromene increased the efficiency of the compound. The outcome revealed that the substitution at the 2-position of the phenyl ring of the chromene moiety (72, 73 and 75) had the least inhibition of bacterial growth.
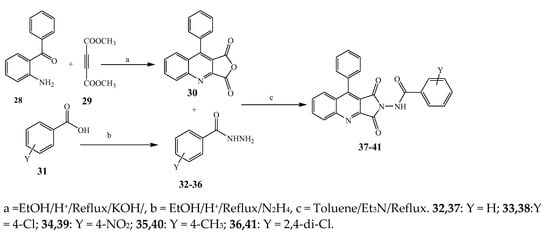
Scheme 2. Synthetic route for the synthesis of pyrrole[3,4-b]quinolin-2(3H)-yl)bezamide derivatives (37–41).
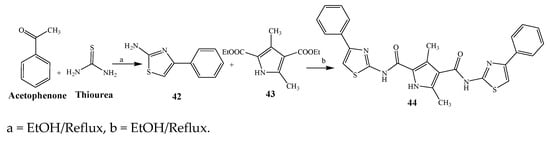
Scheme 3. Synthetic route of compound 44.
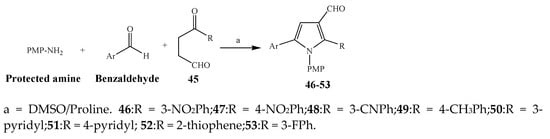
Scheme 4. Synthesis of pyrrole from 1,4-dicarbonyls.
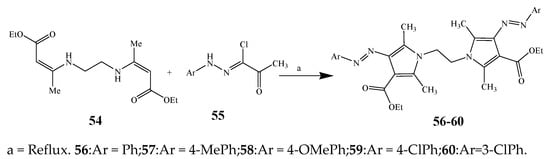
Scheme 5. Synthesis of bis-pyrrole derivatives.
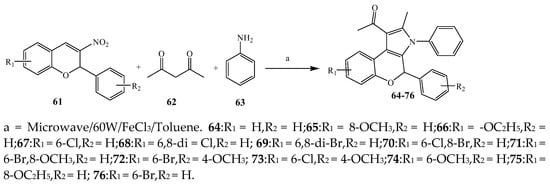
Scheme 6. Synthesis of 2H-chromene fused pyrrole derivatives (64–76).
2.2. Synthesis and Antibacterial Activity of the Pyrimidine
Pyrano[2,3-d]pyrimidine-6-carboxylates (82–90) were synthesized via a multistep reaction (Scheme 7) [24]. The antibacterial activity for the synthesized compounds was checked against Gram-negative (Klebsiella pneumonia, E. coli) and Gram-positive (P. aeruginosa, S. aureus) strains using ciprofloxacin as a standard drug. Compounds 80–81, 83–85, 87, 89 and 90 exhibited exceptional activity, while compounds 83, 86, and 88 showed remarkable activity (Table 1). They further elaborated that compounds with an electron-withdrawing substituent (–OCH3 and –NO2) exhibited maximum activity as compared to compounds with the methyl group [24].
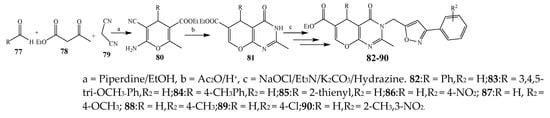
Scheme 7. Synthetic route of pyrimidine from aromatic aldehyde (82–90).
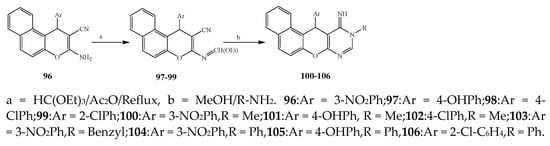
Misra et al., 2018 reported the synthesis of pyrimidine derivatives with 1,5-benzodiazepines under microwave conditions using 1,4-diazabicyclo[2.2.2]octane and dimethyl acetylenedicarboxylate, as shown in Scheme 8. The antibacterial results indicated that compound 95 had exhibited persuasive inhibitory activity against E. coli and S. aureus with IC50 values of 300 and 200 μg/mL, respectively [25]. The synthesis of (benzo[5,6]chromeno[2,3-d]pyrimidine (96–106) (Scheme 9) was reported by Ameli et al., 2017, while their anti-infectious activity was checked against S. aureus, B. subtilis, E. coli and Salmonella typhimurium. Compounds 100–106 showed high activity while 96-99 remained moderate (MIC > 250). These results suggested that the pyrimidine moiety plays a significant role in antibacterial activity. Compounds 101, 102, 105 and 106 whose aryl groups have a Cl or OH group, displayed greater inhibitory effects against E. coli, S. aureus, S. typhimurium and B. subtilis strains which may have the potential to bind the compounds to corresponding receptors [14]. The 2,4-disubstituted-6-thiophenyl-pyrimidine as antibacterial agents was prepared by Fang et al., 2019 and the route of synthesis is presented in Scheme 10. It is estimated that among compounds 116–123, the most potent were 118, 117 and 123 with 5 μg/mL MIC compared to vancomycin and methicillin (Table 1). On the other hand, the inhibitory effect was weak against E. coli which may be due to the lower penetrating capacity of the synthesized compounds to the outer Gram-negative bacterial membrane [26]. The results further showed the presence of the group at the 2nd position of pyrimidine pivotal activity. The cyclo-condensation protocol was also reported for the synthesis of the bis-2-phenylpyrimidines compound from β-ketoester, benzamidine hydrochloride, and dihaloalkanes as shown in Scheme 11 [27]. The in vitro antibacterial activity of the synthesized compounds 126–134 was also studied against Salmonella enterica ATCC 13312, E. coli ATCC 25922, Micrococcus luteus ATCC 29213, B. subtilis ATCC 29213, and S. aureus ATCC 29213. The results showed that compounds 126–134 had good activities towards B. subtilis, S. enterica and M. luteus, while most of the compounds against S. aureus showed a weak response [27]. Pyrimidine (140–149) and oxadiazole (150–159) were prepared by Triloknadh et al., 2018 as shown in Scheme 12 [28]. Among all the synthesized compounds, 150, 154, 155a, and 156 exhibited vigorous activity with 0.0125 to 0.0412 mg/mL values of MIC, which were higher than gentamicin (MIC = 0.0212 to 0.0422 mg/mL). Compound 141 displayed dominant activity across three strains (P. aeruginosa, B. subtilis and S. aureus) with 0.0124, 0.0120, and 0.0311 mg/mL MIC values, respectively (Table 1). Moreover, if the methoxy group was moved from position 3 to position 4 (140), it may lead to the reduction of activity across the strains. In addition, methyl, cyano, and bromo substitutions enhanced the activity. Andrews et al., 2017 described the synthesis of the pyrimidine derivatives (161–170), (171–180) as represented in Scheme 13. The in vitro antibacterial activity of the recently synthesized compounds was screened against E. coli, S. aureus and P. aeruginosa by agar well disk diffusion technique. The antibacterial activity data showed that compounds 161–170 had lower inhibition than compounds 171–180 [29].
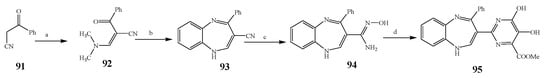
Scheme 8. Synthesis of pyrimidine derivatives of 1,5-benzodiazepine from benzoylacetonitrile.
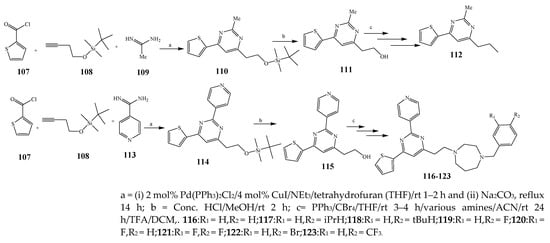
Scheme 10. Synthesis of pyrimidine using acetamidine and isonicotinamidine.
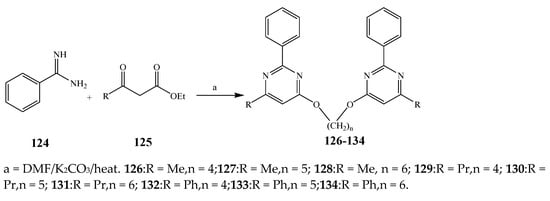
Scheme 11. Synthesis of bis-pyrimidines (126–134).
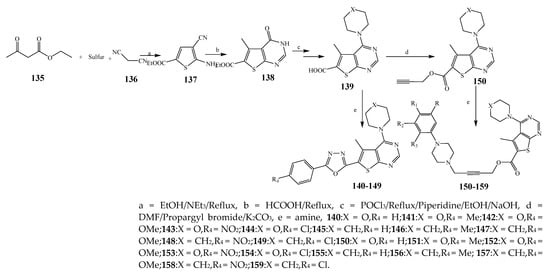
Scheme 12. Synthesis of thieno[2,3-d]pyrimidine-alkyne.
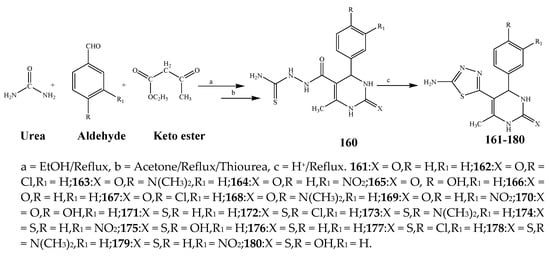
Scheme 13. Synthesis of 3,4-dihydro-5-(5-mercapto-4H-1,2,4-triazol-3-yl)-6-methyl-4-phenyl pyrimidin-2(1H)-one (161–180).
2.3. Synthesis and Antibacterial Activity of the Thiazole
The thiazole derivatives (181–189), such as 3-substituted-4-aryl-2-[(1-phenylethylidene)hydra-zono]-2,3-dihydrothiazoles and 4-Aryl-2-[2-(1-substituted ethylidene) hydra-zinyl]-thiazoles were prepared through the reaction of bromoacetophenones and (ethylidene)hydrazinecarbothioamides (Scheme 14 and Scheme 15) [30]. Compounds 192–209 were evaluated against different Gram-positive and Gram-negative strains of bacteria such as K. pneumonia, P. aeruginosa, S. aureus, and E. coli using reference drugs. The results concluded that compounds 192, 194, 195, 200 and 205 had remarkable activity against E. coli. Furthermore, compounds 195, 192, 196, 200, 209 and 207 reported the promising antibacterial activity against K. pneumonia, which is greater than ciprofloxacin. Compounds 192, 194, 195, 200, 205 and 207 exhibited excellent activity against P. aeruginosa, which was higher as compared to ciprofloxacin. Moreover, 196, 200, 205, 207 and 209 displayed several folds of activity against S. aureus. It was indicated from the results that the existence of a phenyl ring at thiosemicarbazones is necessary for significant activity. Similarly, the allyl, benzyl and phenyl group on the N-position of thiazole (207, 209 and 205) was associated with a significantly high degree of activity [30]. Thirty thiazole derivatives (213–242) were synthesized by Zha et al., 2019 as represented in Scheme 16. In vitro antibacterial studies of compounds were carried out against B. subtilis, S. aureus, K. pneumonia and E. coli. The results explained that compounds 231 and 232 demonstrated valuable activity against all the bacterial strains, while compounds 226 and 227 revealed good activity against E. coli, S. aureus and B. subtilis because of the existence of an electron-donating (-OCH3 and -OH) moiety in the molecule [31]. Similarly, Abdel-Galil et al., 2018 reported the synthesis of a series of scaffolds of heterocyclic compounds that contain thiazolidin-5-one and thiazole rings from 4-formylphenyl benzoate precursor (Scheme 17). Antibacterial activity of the synthesized compounds was performed against S. aureus and E. coli. The results reported that compounds 244, 245 and 248 exhibited dominant antibacterial activity against the S. aureus bacterial strain, while 6 displayed exceptional activity against E. coli. Moreover, compounds 251, 250 and 246 demonstrated acceptable activity across the positive strain and compounds 244 and 247 revealed good activity against the negative strain (Table 1). The existence of ethoxy, methyl, amino, azo, hydroxyl, or methoxy groups assimilated with phenylbenzoate, cyanoacetyl hydrazine, and carbamothioylhydrazone moiety in the derivatives of thiazole depicted excellent antibacterial activity. Furthermore, the presence of an electron-withdrawing group rather than an electron-donating group in compound 249 reduced the activity. The compound 248 showed the excellent antibacterial activity against S. aureus and E. coli bacterial strains, which may be due to the –CH3 group at the 4th position of thiazole [32]. The synthesis of thiazoles from allyl thiourea as given in Scheme 18 was carried out by Khare, et al., 2016. All the synthesized compounds (253–258) exhibited moderate activity [33]. Beyzaei, et al., 2017 illustrated the synthesis of 4-thiazolylpyrazole (264–269) compounds from the modified Hantzsch method, in the presence of catalyst MgO nanoparticles as represented in Scheme 19. An evaluation of the in vitro antibacterial activity of the synthesized compounds was also performed against 21 bacteria. Compounds 264 to 269 of 4-thiazolylpyrazoles exhibited inhibition activities against S. agalactiae, P. aeruginosa and S. pneumoniae. In comparison with the other compounds, thiazoles 265 and 269 were potent against eight pathogenic bacterial strains, and as such they appeared as one of the effective broad-spectrum agents [34]. Mohamed et al., 2018 reported the synthesis of thiazole derivatives as shown in Scheme 20 and observed that the derivative of 272 had greater antibacterial activity. This may be due to the thiazole’s molecular structure and also the existence of the NH group, which helps the adsorption of a compound onto the surface of bacteria, perforation into their cell membrane, and finally eradication of the membrane, causing the death of bacteria [35]. Seven unique derivatives of pleuromutilin with the moiety of thioether and thiazole-5-carboxamide (277–283) were prepared by Wang et al., 2011 as represented in Scheme 21. The in vitro antibacterial activity of the synthesized compounds was evaluated against two positive strains of bacteria viz. S. aureus ATCC26112 and S. aureus SC. The compounds exhibited valuable activity against the bacteria and it can be examined that the bacterial strain of S. aureus was more susceptible than S. aureus [36].
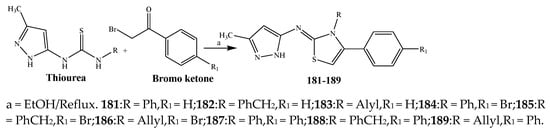
Scheme 14. (Thiazol-2-yl)pyrazol-5-amines from pyrazolylthioureas and ω-bromoacetophenones.
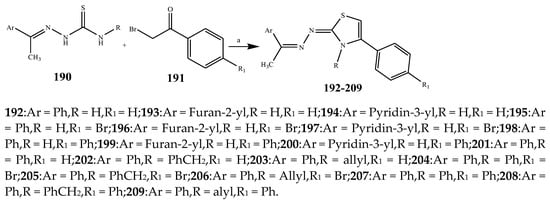
Scheme 15. Synthetic route of compounds (192–209).
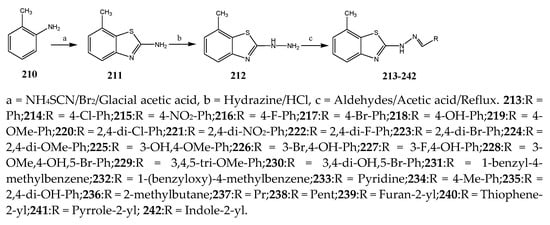
Scheme 16. Synthetic routes of various thiazoles.
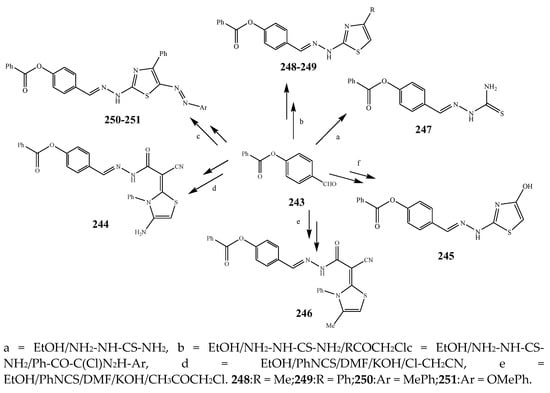
Scheme 17. Synthetic routes of thiazole.
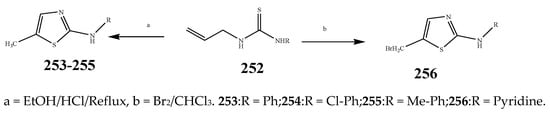
Scheme 18. Synthesis of thiazole from thiourea (253–256).
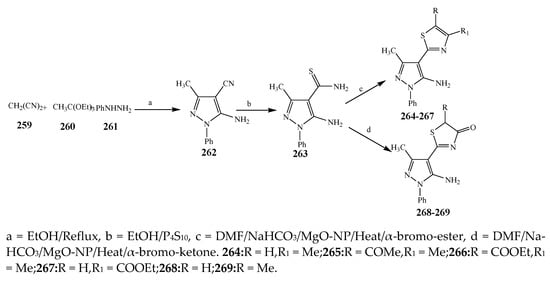
Scheme 19. Total synthesis of 4-thiazolylpyrazoles.
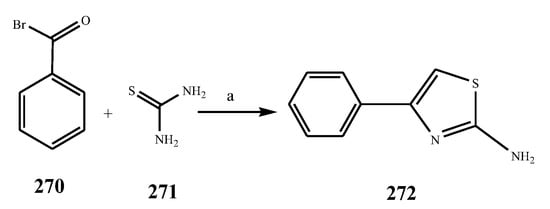
Scheme 20. Synthetic routes of substituted thiazole.
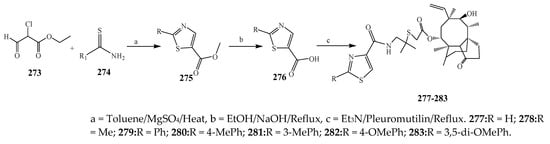
Scheme 21. The synthetic route to thiazole having pleuromutilin moiety.
Dear authors, as there is a word limit for us drafting, please kindly add the left content of this section once you decide to submit to Encyclopedia, thank you.
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics11121750
This entry is offline, you can click here to edit this entry!