Intervertebral disc degeneration (IDD), a progressive and multifactorial pathological process, is predominantly associated with low back pain and permanent disability. Pyroptosis is a type of lytic programmed cell death triggered by the activation of inflammasomes and caspases. Unlike apoptosis, pyroptosis is characterized by the rupture of the plasma membrane and the release of inflammatory mediators, accelerating the destruction of the extracellular matrix (ECM). Recent studies have shown that pyrin domain-containing 3 (NLRP3) inflammasome-mediated pyroptosis in nucleus pulposus (NP) cells is activated in the progression of IDD. Furthermore, targeting pyroptosis in IDD demonstrates the excellent capacity of ECM remodeling and its anti-inflammatory properties, suggesting that pyroptosis is involved in the IDD process. Here, the molecular mechanism of pyroptosis and the pathogenesis of IDD are briefly summarized. Researchers also focus on the role of pyroptosis in the pathological progress of IDD and its targeted therapeutic application.
- pyroptosis
- intervertebral disc degeneration
- NLRP3 inflammasome
- nucleus pulposus
1. Pyroptosis Triggers Cell Death in IDD
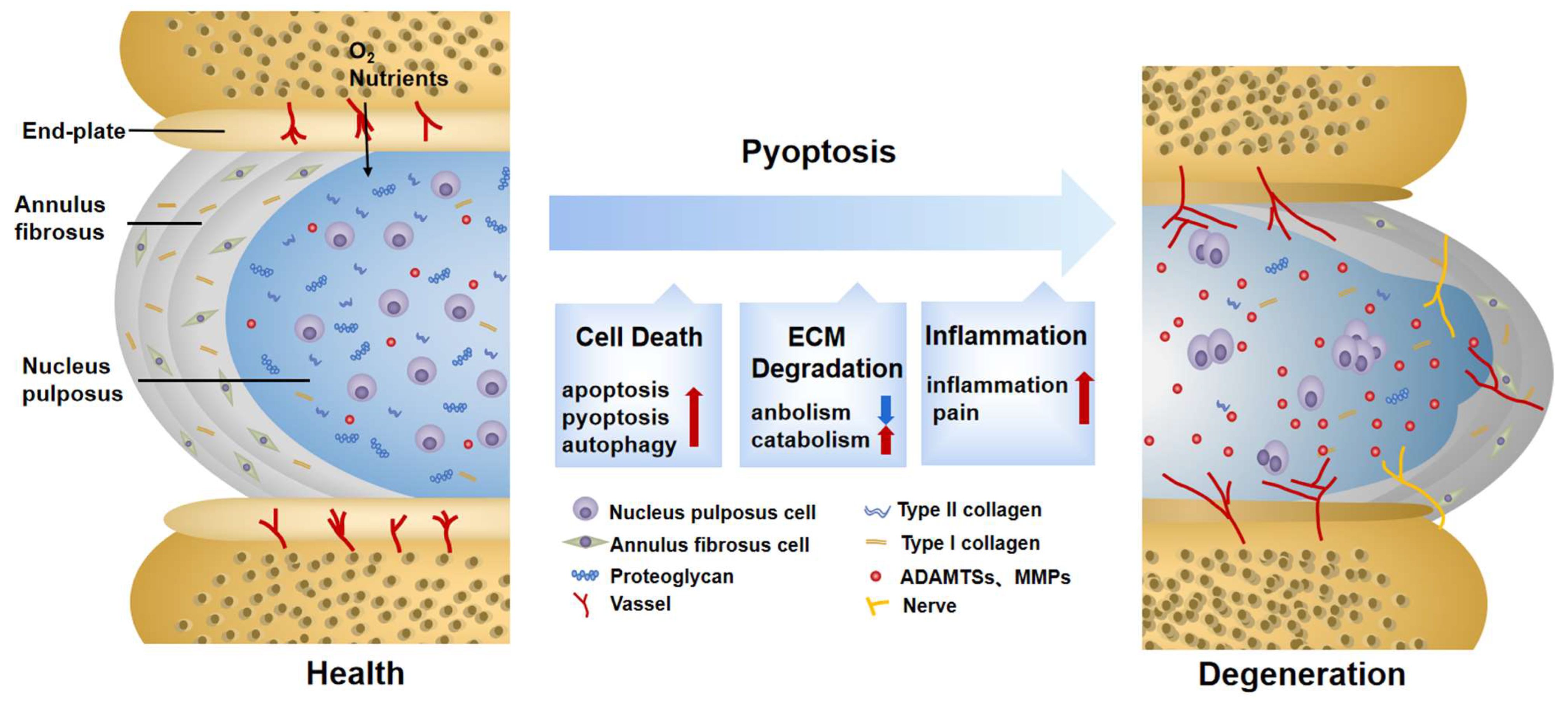
2. Pyroptosis Provokes ECM Disorder in IDD
3. Pyroptosis Induces Secondary Inflammation in IDD
This entry is adapted from the peer-reviewed paper 10.3390/biom12121804
References
- Ding, F.; Shao, Z.W.; Xiong, L.M. Cell death in intervertebral disc degeneration. Apoptosis 2013, 18, 777–785.
- Chen, Z.H.; Jin, S.H.; Wang, M.Y.; Jin, X.L.; Lv, C.; Deng, Y.F.; Wang, J.L. Enhanced NLRP3, caspase-1, and IL- 1beta levels in degenerate human intervertebral disc and their association with the grades of disc degeneration. Anat. Rec. 2015, 298, 720–726.
- Fu, F.; Bao, R.; Yao, S.; Zhou, C.; Luo, H.; Zhang, Z.; Zhang, H.; Li, Y.; Yan, S.; Yu, H.; et al. Aberrant spinal mechanical loading stress triggers intervertebral disc degeneration by inducing pyroptosis and nerve ingrowth. Sci. Rep. 2021, 11, 772.
- Peng, X.; Zhang, C.; Zhou, Z.M.; Wang, K.; Gao, J.W.; Qian, Z.Y.; Bao, J.P.; Ji, H.Y.; Cabral, V.L.F.; Wu, X.T. A20 attenuates pyroptosis and apoptosis in nucleus pulposus cells via promoting mitophagy and stabilizing mitochondrial dynamics. Inflamm. Res. 2022, 71, 695–710.
- Wang, Y.; Kanneganti, T.D. From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways. Comput. Struct. Biotechnol. J. 2021, 19, 4641–4657.
- Li, Y.; Wu, X.; Li, J.; Du, L.; Wang, X.; Cao, J.; Li, H.; Huo, Z.; Li, G.; Pan, D.; et al. Circ_0004354 might compete with circ_0040039 to induce NPCs death and inflammatory response by targeting miR-345-3p-FAF1/TP73 axis in intervertebral disc degeneration. Oxid. Med. Cell Longev. 2022, 2022, 2776440.
- Li, Y.; Yuan, Y.; Huang, Z.X.; Chen, H.; Lan, R.; Wang, Z.; Lai, K.; Chen, H.; Chen, Z.; Zou, Z.; et al. GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy. Cell Death Differ. 2021, 28, 2333–2350.
- Hong, J.; Li, S.; Markova, D.Z.; Liang, A.; Kepler, C.K.; Huang, Y.; Zhou, J.; Yan, J.; Chen, W.; Huang, D.; et al. Bromodomain-containing protein 4 inhibition alleviates matrix degradation by enhancing autophagy and suppressing NLRP3 inflammasome activity in NP cells. J. Cell Physiol. 2020, 235, 5736–5749.
- Chen, J.; Yan, J.; Li, S.; Zhu, J.; Zhou, J.; Li, J.; Zhang, Y.; Huang, Z.; Yuan, L.; Xu, K.; et al. Atorvastatin inhibited TNF-alpha induced matrix degradation in rat nucleus pulposus cells by suppressing NLRP3 inflammasome activity and inducing autophagy through NF-kappaB signaling. Cell Cycle 2021, 20, 2160–2173.
- Liao, Z.; Li, S.; Liu, R.; Feng, X.; Shi, Y.; Wang, K.; Li, S.; Zhang, Y.; Wu, X.; Yang, C. Autophagic Degradation of Gasdermin D Protects against Nucleus Pulposus Cell Pyroptosis and Retards Intervertebral Disc Degeneration In Vivo. Oxid. Med. Cell Longev. 2021, 2021, 5584447.
- Le Maitre, C.L.; Pockert, A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 2007, 35, 652–655.
- Huang, Y.; Peng, Y.; Sun, J.; Li, S.; Hong, J.; Zhou, J.; Chen, J.; Yan, J.; Huang, Z.; Wang, X.; et al. Nicotinamide Phosphoribosyl Transferase Controls NLRP3 Inflammasome Activity through MAPK and NF-kappaB Signaling in Nucleus Pulposus Cells, as Suppressed by Melatonin. Inflammation 2020, 43, 796–809.
- Yan, J.; Li, S.; Zhang, Y.; Deng, Z.; Wu, J.; Huang, Z.; Qin, T.; Xiao, Y.; Zhou, J.; Xu, K.; et al. Cholesterol Induces Pyroptosis and Matrix Degradation via mSREBP1-Driven Endoplasmic Reticulum Stress in Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2021, 9, 803132.
- Lawson, L.Y.; Harfe, B.D. Developmental mechanisms of intervertebral disc and vertebral column formation. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e283.
- Tang, G.; Han, X.; Lin, Z.; Qian, H.; Chen, B.; Zhou, C.; Chen, Y.; Jiang, W. Propionibacterium acnes Accelerates Intervertebral Disc Degeneration by Inducing Pyroptosis of Nucleus Pulposus Cells via the ROS-NLRP3 Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 4657014.
- Wang, W.J.; Yu, X.H.; Wang, C.; Yang, W.; He, W.S.; Zhang, S.J.; Yan, Y.G.; Zhang, J. MMPs and ADAMTSs in intervertebral disc degeneration. Clin. Chim. Acta 2015, 448, 238–246.
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The role of IL-1beta and TNF-alpha in intervertebral disc degeneration. Biomed. Pharmacother. 2020, 131, 110660.
- Zhao, K.; An, R.; Xiang, Q.; Li, G.; Wang, K.; Song, Y.; Liao, Z.; Li, S.; Hua, W.; Feng, X.; et al. Acid-sensing ion channels regulate nucleus pulposus cell inflammation and pyroptosis via the NLRP3 inflammasome in intervertebral disc degeneration. Cell Prolif. 2021, 54, e12941.
- Brand, F.J., 3rd; Forouzandeh, M.; Kaur, H.; Travascio, F.; de Rivero Vaccari, J.P. Acidification changes affect the inflammasome in human nucleus pulposus cells. J. Inflamm. 2016, 13, 29.
- Song, Y.; Wang, Y.; Zhang, Y.; Geng, W.; Liu, W.; Gao, Y.; Li, S.; Wang, K.; Wu, X.; Kang, L.; et al. Advanced glycation end products regulate anabolic and catabolic activities via NLRP3-inflammasome activation in human nucleus pulposus cells. J. Cell Mol. Med. 2017, 21, 1373–1387.
- Johnson, Z.I.; Schoepflin, Z.R.; Choi, H.; Shapiro, I.M.; Risbud, M.V. Disc in flames: Roles of TNF-alpha and IL-1beta in intervertebral disc degeneration. Eur. Cell Mater. 2015, 30, 104–116; discussion 107–116.
- Capossela, S.; Schlafli, P.; Bertolo, A.; Janner, T.; Stadler, B.M.; Potzel, T.; Baur, M.; Stoyanov, J.V. Degenerated human intervertebral discs contain autoantibodies against extracellular matrix proteins. Eur. Cell Mater. 2014, 27, 251–263; discussion 263.
- Sun, Z.; Liu, B.; Luo, Z.J. The Immune Privilege of the Intervertebral Disc: Implications for Intervertebral Disc Degeneration Treatment. Int. J. Med. Sci. 2020, 17, 685–692.
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56.
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541.
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther. 2005, 7, R732–R745.
- Jimbo, K.; Park, J.S.; Yokosuka, K.; Sato, K.; Nagata, K. Positive feedback loop of interleukin-1beta upregulating production of inflammatory mediators in human intervertebral disc cells in vitro. J. Neurosurg. Spine 2005, 2, 589–595.
- Penolazzi, L.; Bergamin, L.S.; Lambertini, E.; Poma, V.V.; Sarti, A.C.; De Bonis, P.; Di Virgilio, F.; Piva, R. The P2X7 purinergic receptor in intervertebral disc degeneration. J. Cell Physiol. 2022, 237, 1418–1428.
- Chen, Z.; Cao, P.; Zhou, Z.; Yuan, Y.; Jiao, Y.; Zheng, Y. Overview: The role of Propionibacterium acnes in nonpyogenic intervertebral discs. Int. Orthop. 2016, 40, 1291–1298.
- He, D.; Zhou, M.; Bai, Z.; Wen, Y.; Shen, J.; Hu, Z. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell pyroptosis via NLRP3-dependent pathway. Biochem. Biophys. Res. Commun. 2020, 526, 772–779.
- Stefanakis, M.; Al-Abbasi, M.; Harding, I.; Pollintine, P.; Dolan, P.; Tarlton, J.; Adams, M.A. Annulus fissures are mechanically and chemically conducive to the ingrowth of nerves and blood vessels. Spine 2012, 37, 1883–1891.
- Johnson, W.E.; Sivan, S.; Wright, K.T.; Eisenstein, S.M.; Maroudas, A.; Roberts, S. Human intervertebral disc cells promote nerve growth over substrata of human intervertebral disc aggrecan. Spine 2006, 31, 1187–1193.
- Arkless, K.; Argunhan, F.; Brain, S.D. CGRP Discovery and Timeline. Handb. Exp. Pharmacol. 2019, 255, 1–12.
- Zhang, A.; Wang, K.; Ding, L.; Bao, X.; Wang, X.; Qiu, X.; Liu, J. Bay11-7082 attenuates neuropathic pain via inhibition of nuclear factor-kappa B and nucleotide-binding domain-like receptor protein 3 inflammasome activation in dorsal root ganglions in a rat model of lumbar disc herniation. J. Pain Res. 2017, 10, 375–382.
- Sun, Y.; Leng, P.; Song, M.; Li, D.; Guo, P.; Xu, X.; Gao, H.; Li, Z.; Li, C.; Zhang, H. Piezo1 activates the NLRP3 inflammasome in nucleus pulposus cell-mediated by Ca2+/NF-kappaB pathway. Int. Immunopharmacol. 2020, 85, 106681.
- Mamet, J.; Lazdunski, M.; Voilley, N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J. Biol. Chem. 2003, 278, 48907–48913.
