1. DKA in Pregnancy
DKA in pregnancy is poorly understood condition due to limited published evidence of risk factors and outcomes. DKA in pregnancy is an obstetric and medical emergency for both the pregnant woman and the fetus and therefore requires prompt and aggressive treatment [
21]. Fetal and neonatal morbidity can be the direct consequence of the extremely poor tolerance of the fetus to acidosis, resulting in intrauterine death or preterm delivery. DKA in pregnancy can occur at near normal levels of blood glucose (euglycemic DKA) and often progresses more rapidly as compared with nonpregnancy [
22]. Prior studies identified number of risk factors that can lead to precipitating episodes of diabetic ketoacidosis in pregnant patients. The major risk factors are intractable vomiting and starvation (53%), inadequate insulin management (18%), and infection (27%) including pyelonephritis, respiratory, chorioamnionitis, ear infection, cellulitis, or tooth abscess. Additional common causes include the use of β-sympathomimetic drugs, steroid administration for fetal lung maturation) and diabetic gastroparesis [
21]. Management of DKA during pregnancy is similar to DKA in non-pregnant patients. Initial fluid replacement starts with 0.9% sodium chloride (normal saline) at a rate of 15 to 20 mL/kg (−1–1.5 L) over the first hour then the rate and type of fluids are determined accordingly. The clinical assessment of the mother involves blood pressure, pulse, hydration, mental status, fluid input and urine output. Additionally, fetal heart rate monitoring is recommended for a gestational age of 24 weeks or more [
23].
2. DKA in Renal Replacement
Diabetes is one of the major precipitating factors of end-stage renal disease (ESRD) in the US [
24]. A normal renal function is integral to maintain insulin homeostasis including its secretion, clearance and level of sensitivity. Hence, impairment or loss of renal function presents multiple challenges in glycemic control for ESRD-patients. Driven mainly by accumulated uremic toxins, ESRD can increase hepatic gluconeogenesis reduce utilization of insulin peripherally and drive insulin resistance [
25]. Interestingly, DKA is uncommon in ESRD-patients possibly because of the absence of glycosuria and osmotic diuresis. Clinical and biochemical lab presentation of DKA in ESRD-dialysis patients is different when compared to non-dialysis patients [
26]. For instance, patients with ESRD present with higher admission blood glucose, volume overload and need for mechanical ventilation [
27]. Interestingly, ESRD-patients have lower A1c, similar mortality, but longer length of stay and higher hospital costs [
27].
Overall, ESRD dialysis patients maintain normal intracellular and extracellular volume. Therefore, they present with minimal volume depletion, and instead they might experience edema of lungs and lower extremities as well as elevated blood pressure; collectively signs of volume expansion. Traditionally, dialysis patients do not require intravenous fluids unless patients exhibit clinical signs of extracellular fluid loss such as vomiting or diarrhea. If needed, small boluses of normal saline (250 mL) would be administered under tight monitoring to maintain stable hemodynamic parameters. Hemodialysis is reserved for severe hyperkalemia and pulmonary edema and continues to be controversial in general. Hyperkalemia in ESRD can be life threatening and could be attributed to reduced glomerular filtration rate, lack of insulin, and hypertonicity [
28]. Severe hyperkalemia requires close monitoring for signs of cardiac toxicity and detection of potassium level post hemodialysis.
3. DKA in Acute Pancreatitis and Islets Transplants
Acute pancreatitis (AP) is one of the common causes of acute abdominal pain related hospital admission with high morbidity and mortality [
29]. AP is associated mostly with hyperlipidemia, uncontrolled diabetes, alcoholism and is seen more with patients with familial hyperlipidemia [
30]. Several reports showed the incidence of severe DKA in patients with AP that was associated with hypertriglyceridemia [
29,
31,
32,
33,
34]. It is difficult to establish a causal relationship whether DKA is the cause or the result of AP, DKA was managed using standard fluid resuscitation and insulin infusion in these patients.
With the success of organ transplants, wide use of immunosuppressants including tacrolimus can cause drug-induced pancreatic islet cell damage and subsequent decrease in insulin secretion [
35]. This can lead to post-transplant diabetes mellitus and diabetic ketoacidosis [
36]. Recent report documented a case of tacrolimus-induced acute pancreatitis in association with hypertriglyceridemia and DKA post-kidney transplant [
37]. Pancreatic islet transplantation is becoming a potential approach to β-cell replacement therapy and the treatment of insulin-deficient diabetes in the setting of recurrent AP or chronic pancreatitis [
38]. Additionally, recurrent episodes of DKA in patients with type-1 diabetes is one of the considerations for islet transplantation [
39]. Islet auto-transplantation involves infusion of islets isolated from the diseased pancreas via the portal vein and intrahepatic engraftment. Tacrolimus remains one of the maintenance immunosuppression for islet transplant recipients with close monitoring for glucose levels and insulin secretions in these patients [
40]. Despite the success of islet transplantation in improving insulin-requirements in type-1 diabetes, it comes with a list of limitations mainly associated with sustained use of immunosuppressants [
38]. As such, it should be saved for patients with type-1 diabetes in whom other, less invasive current treatments have been repeatedly ineffective.
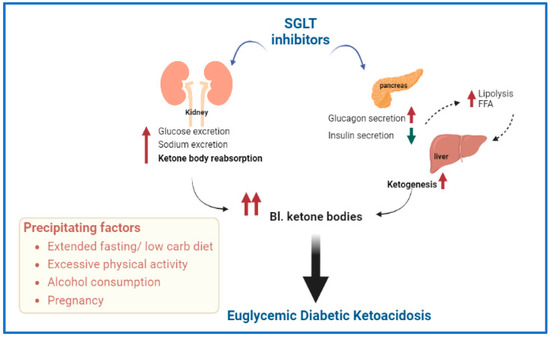
4. DKA and Sodium–Glucose Cotransporter (SGLT) Inhibitors
SGLT2 inhibitors including Canagliflozin, Dapagliflozin, Empagliflozin and Ertugliflozin are popular in the US and Europe as their use is approved in patients with type-2 diabetes as a first line therapy. Further, they have been studied and used off-label in combination with insulin therapy in patients with type 1 diabetes [
41]. The use of SGLT2 inhibitors has been increasingly associated with an increased risk of DKA primarily in patients with type-1 diabetes and to a lesser extent in type-2 diabetes. Blocking SGLT2 transporter in the proximal tubule of the kidney results in glycosuria and natriuresis. SGLT-2 inhibition results in excretion of 50–60% of the filtered glucose, but also exerts a mild natriuretic effect that provide additional benefits including weight loss and reduction in systolic/diastolic blood pressure [
42]. SGLT-2 inhibitors are potent cardioprotective and nephroprotective agents, offering reductions of up to 38% in cardiovascular mortality, 35% in heart failure hospitalization, 45% in progression of renal disease, and 30% in all-cause mortality (reviewed in [
43]). The ability of SGLT2 inhibitors to induce osmotic diuresis allows the body to get rid of extra free water and supported potential use of SGLT2 inhibitors in cases of euvolemic hyponatremia [
44,
45]. While this particular use warrant further studies, a recent case review showed that SGLT2 inhibitors can cause hyponatremia as a potential, rare side effect and that the use of SGLT2 inhibitor should be evaluated or stopped in case of persistent hyponatremia [
46].
Development of euglycemic DKA and ketonemia remain the most dangerous side effects for patients who develop DKA while on SGLT inhibitor therapy [
47]. SGLT2 inhibitors can induce a state of ketosis by increasing both urinary glucose excretion and reabsorption of ketone bodies. Precipitating factors include prolonged periods of fasting, reduced carbohydrate intake, excessive physical activity, infection, pregnancy or alcohol consumption [
48]. These factors can accelerate lipolysis and increased free fatty acid levels that eventually trigger hepatic formation of ketone bodies (see
Figure 3).
Figure 3. Schematic representation of possible mechanisms involved in the development of euglycemic diabetic ketoacidosis (DKA) associated with sodium glucose transporter (SGLT) inhibitors. Precipitating factors are also included. Abbreviations—FFA: free fatty acids, Bl.: blood.
SGLT2 inhibitors are approved for type 1 diabetes in Europe and Japan, with off-label use in type-1 diabetes in the US [
49]. Due to mild hyperglycemia associated with SGLT use, DKA diagnosis can be missed or delayed. Therefore, there is a need for patients to be educated regarding how to recognize and reduce elevated ketone levels. Development of unusual symptoms of physical sickness such as nausea, vomiting, abdominal pain, fatigue or malaise in these patients should be recognized. Recognizing these symptoms should prompt measuring ketones in the blood (preferred) or urine to evaluate for DKA [
50]. A number of protocols have been developed to treat DKA for individuals who are treated with SGLT2 inhibitors. One of them is the STOP DKA protocol (Symptomatic to stop SGLT inhibitor, Test ketones and glucose, Oral ingestion of fluids and carbohydrates, Protocol instructions for insulin and carbohydrates) [
50]. STOP DKA defines mild ketonemia as <1.0 mmol/L vs. 0.6 mmol/L in most of the other protocols. SGLT2 inhibitors should be stopped as soon as any symptoms of physical illness (e.g., lethargy, loss of appetite, nausea, or abdominal pain) or elevated ketone levels are detected and resumed only after ketone levels return to normal. Generally, patients should consume carbohydrates and give insulin in an attempt to lower ketone levels. If levels of ketones are elevated then the STICH (STop SGLT inhibitor, Insulin administration, Carbohydrate consumption, Hydration) protocol should be initiated [
51]. After stopping SGLT2 inhibitor therapy, patients should receive rapid- or short-acting insulin and consume 15–30 g of rapidly absorbed carbohydrate such as juice, soda, or milk along with 300–500 mL of fluid. The level of ketones should be monitored every 3–4 h until resolution back to normal. Comparatively, the STOP DKA protocol provides specific insulin recommendations based on blood glucose and ketone levels, while the STICH protocol recommends that patients take 1.5 times their usual dose of insulin.
5. DKA in Patients with Congestive Heart Failure (CHF)
Similar to patients with chronic kidney disease, specific population considerations should be given to patients with CHF [
10]. These patients tend to retain fluids; therefore, volume resuscitation should be carefully titrated in patient with CHF. A conservative fluid resuscitation is crucial in the management of the DKA patient with CHF. Minimal fluid bolus, such as (250–500 mL) is recommended in hypovolemic patients with subsequent monitoring for signs/symptoms of volume overload. Intravenous insulin is the main stay to improve acidosis by preventing ketone production [
25]. Further, urine output monitoring is an important step in patients with hyperglycemic crises.
6. DKA and Insulin Pump Users
Early studies showed that insulin pump users commonly known as continuous subcutaneous insulin infusion are more prone to development of DKA [
55]. Insulin pump users rely on rapid or short acting insulin, but not the long acting insulin. Hence, if the pump fails or the canula blocked or dislodged, the insulin delivery will be stopped and blood glucose levels rise triggering ketoacidosis to develop rapidly. The prevalence of DKA in users of insulin pump therapy is decreasing most likely due to significant improvements of technology [
56]. Of note, impact of patient training and education is most evident by improved glycemic control, and fewer of episodes of hypoglycemia and DKA after 1-year of pump use [
56].
For the management of possible DKA associated with insulin pump therapy, first the pump-related problem should be identified and rectified. For example, re-calibrating the pump, re-siting the cannula, and changing the tubing should be part of patient education. Switching insulin pump to alternative insulin such as an intravenous infusion can be useful because of altered insulin absorption and tissue perfusion [
57]. If a pump user develops DKA, insulin pump should be immediately discontinued and standard DKA treatment started. Once DKA has resolved, insulin pump therapy can be restarted at the patient’s usual basal rate along with transitional dose of intravenous insulin infusion until a bolus is given via pump [
58].
7. DKA in Patients with COVID-19
During the SARS-CoV-2 commonly referred to as COVID-19 pandemic, it has been established that diabetes is a frequent comorbidity and DKA has been documented in patients with COVID-19 in both patients with T1D and patients with T2D [
59]. There is evidence that support a possible bidirectional relationship between SARS-CoV2 infection and diabetes, with infection being associated with worsening of hyperglycemia in preexisting diabetes and new-onset hyperglycemia [
60]. Nevertheless, there are insufficient data to determine if DKA is more prevalent in COVID-19 and if the SARS-CoV-2 virus poses an increased risk over other severe infectious diseases. To date, only 1 study has described the prevalence of acidosis and ketoacidosis in 658 hospitalized patients with confirmed COVID-19 [
61]. In the same study, patients with ketosis were about twice as likely to have diabetes at baseline, and the 3 individuals who developed DKA had underlying diabetes (1 with T1D, 2 with T2D). Ketosis was also associated with higher mortality. A more recent metanalysis showed that DKA is not uncommon in COVID-19 patients with diabetes mellitus and results in a mortality rate of 25.9% [
62]. The major determinants of mortality in DKA patients with COVID-19 infection are pre-existing diabetes mellitus type 2, older age [≥60 years old], male gender, BMI ≥ 30, blood glucose level > 1000 mg/dL, and anion gap ≥ 30 mEq/L [
62].
One of the important and common finding among COVID-19 patients is that severe disease is accompanied by high levels of inflammatory markers, which are also elevated in the setting of DKA independent of accompanying illness. IL-6 has been highlighted as likely playing a role in a maladaptive immune response to the SARS-CoV-2 virus and has been proposed as a possible treatment target [
63]. Interestingly, IL-6 has also been also found to be elevated in patients with DKA and is thought to be mostly a driver of ketosis rather than a result [
64]. Future studies are warranted to explore the role of inflammation in DKA and COVID-19 in relation to clinical outcomes.
This entry is adapted from the peer-reviewed paper 10.3390/endocrines3040066