In native periodontal ligament (PDL) development and homeostasis, mechanical loading plays a crucial role. Thus, any strategy aiming at periodontal tissue engineering should entail understanding and replicating in vitro the specific mechanical forces that guide the formation and remodeling of the PDL.
- mechanical loading
- mechanotransduction
- periodontal ligament regeneration
- tissue engineering
- bioreactor
- 3D environment
1. PDL Structure and Cells
The periodontium is a complex system composed of gingiva, periodontal ligament (PDL), cementum, and alveolar bone, featuring a hierarchically compartmentalized architecture [1]. The homeostasis of this system is maintained by the PDL, a specialized connective tissue, which is located between the cementum and alveolar bone and articulates (gomphosis) the teeth to the jaws [2][3]. From a histological perspective, PDL is an aligned fibrous network with a thickness ranging between 100 and 400 μm and is characterized by an extensive blood supply and a neural network [4]. PDL is constituted by a heterogeneous population of cells (namely PDL cells) that includes periodontal ligament fibroblasts (PDLFs), which represent by far the largest population and are responsible for the deposition and maintenance of the extracellular matrix (ECM) and periodontal ligament stem cells (PDLSCs), showing both osteogenic and tendo/ligamentogenic characteristics. Collagen type I and, in lesser amounts, type III constitute cross‐banded fibrils, named Sharpey’s fibers, which provide mechanical support and are usually classified as dentinogingival, transseptal, or alveolodental (forming the bulk of proper PDL fibers) [4]. In particular, fibers oblique or perpendicular to the long axis of the tooth are thought to play pivotal roles in eliciting adaptive responses during mastication and occlusion [4]. Among all the fibers, the horizontal ones withstand the greatest loads and exhibit the greatest strain under mastication [5], (Figure 1). The collagen fibers are generally aligned according to a periodic crimped pattern [6] that prevents ligament overextension [7][8]. Sharpey’s fibers anchor mostly to acellular cementum, a mineralized layer (50–300 μm thickness) covering the tooth dentin surface. PDL cells are arranged along PDL fibers so that the long cellular axis is parallel to the main fiber bundles of the PDL [9][10]. The presence of a particular type of elastic fibers named oxtytalan, made of fimbrillins, that form a network running parallel to cementum and are thought to interact with vessels and neural fibers is also noteworthy [11]. In a healthy subject, PDL covers the tooth root almost entirely, and a tight epithelial seal within the gingival sulcus prevents microorganisms from reaching the PDL. This delicate system is compromised by the onset of periodontal disease (PD), which affects in its severe form about 10% of adults, ranking sixth among the most prevalent diseases in the world [12]. PD starts as a localized and reversible inflammation of the gingiva (gingivitis) due to dental plaque, and, when untreated, it may become chronic periodontitis, which is characterized by the progressive destruction of the tooth‐supporting tissues, i.e., cementum, PDL, and bone [13][14].
2. Mimicking the Physical Micro-Environment of PDL
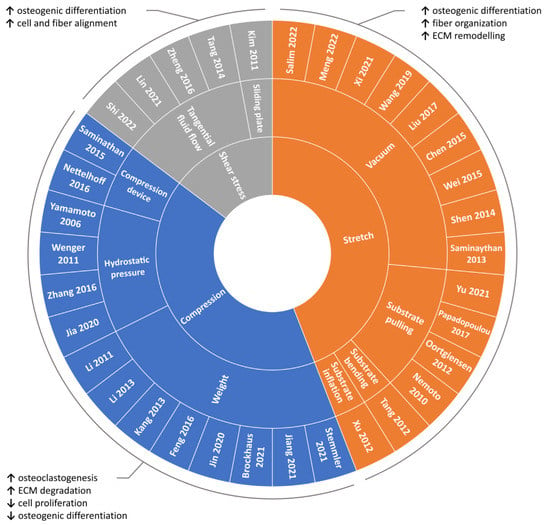
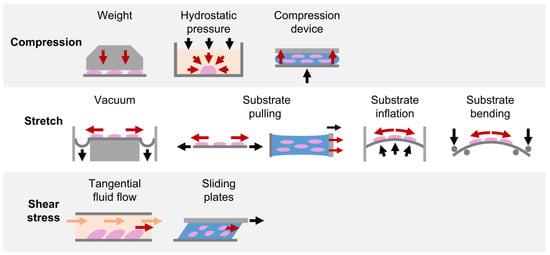
2.1. Compression
2.1.1. Weight Method
2.1.2. Hydrostatic Pressure Method
2.1.3. Substrate Deformation Methods
2.2. Stretch
2.2.1. Substrate Deformation—Vacuum Approach
2.2.2. Substrate Deformation—Pulling Approach
2.2.3. Substrate Deformation—Inflation and Bending Approaches
2.3. Shear Stress
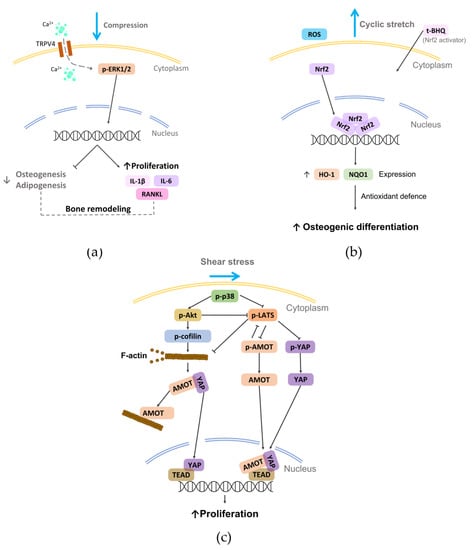
The above-mentioned findings highlight how shear stress can represent an effective approach for guiding cell and fiber alignment and for promoting osteogenic differentiation.
This entry is adapted from the peer-reviewed paper 10.3390/nano12213878
References
- Menicanin, D.; Hynes, K.; Han, J.; Gronthos, S.; Bartold, P.M.; Cementum and Periodontal Ligament Regeneration. Adv. Exp. Med. Biol. 2015, 881, 207–236, 10.1007/978-3-319-22345-2_12.
- Socransky, S.S.; Haffajee, A.D.; Periodontal microbial ecology. Periodontology 2000 2005, 38, 135–187, 10.1111/j.1600-0757.2005.00107.x.
- Beertsen, W.; McCulloch, C.A.; Sodek, J.; The periodontal ligament: A unique, multifunctional connective tissue. Periodontology 2000 1997, 13, 20–40, 10.1111/j.1600-0757.1997.tb00094.x.
- de Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H.; The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration.. J. Periodontal Res 2017, 52, 965–974, 10.1111/jre.12477.
- Ortun-Terrazas, J.; Cegonino, J.; Santana-Penin, U.; Santana-Mora, U.; Perez Del Palomar, A.; Approach towards the porous fibrous structure of the periodontal ligament using micro-computerized tomography and finite element analysis. J. Mech. Behav. Biomed. Mater. 2018, 79, 135–149, 10.1016/j.jmbbm.2017.12.022.
- Gathercole, L.J.; Keller, A.; Crimp morphology in the fibre-forming collagens. Matrix 1991, 11, 214–234, 0.1016/s0934-8832(11)80161-7.
- Maceri, F.; Marino, M.; Vairo, G.; A unified multiscale mechanical model for soft collagenous tissues with regular fiber arrangement.. J. Biomech. 2010, 43, 355–363, 10.1016/j.jbiomech.2009.07.040.
- Szczesny, S.E.; Driscoll, T.P.; Tseng, H.Y.; Liu, P.C.; Heo, S.J.; Mauck, R.L.; Chao, P.G.; Crimped Nanofibrous Biomaterials Mimic Microstructure and Mechanics of Native Tissue and Alter Strain Transfer to Cells. ACS Biomater. Sci. Eng. 2017, 3, 2869–2876, 10.1021/acsbiomaterials.6b00646.
- Cho, M.I.; Garant, P.R.; Development and general structure of the periodontium.. Periodontology 2000 2000, 24, 9–27, 10.1034/j.1600-0757.2000.2240102.x.
- Bosshardt, D.D.; Bergomi, M.; Vaglio, G.; Wiskott, A.; Regional structural characteristics of bovine periodontal ligament samples and their suitability for biomechanical tests. J. Anat. 2008, 212, 319–329, 10.1111/j.1469-7580.2008.00856.x.
- Tsuruga, E.; Irie, K.; Sakakura, Y.; Yajima, T.; Expression of fibrillins and tropoelastin by human gingival and periodontal ligament fibroblasts in vitro. J. Periodontal Res. 2002, 37, 23–28, 10.1034/j.1600-0765.2002.00662.x.
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge.. Lancet 2019, 394, 249–260, 10.1016/S0140-6736(19)31146-8.
- Lourenco, T.G.; Heller, D.; Silva-Boghossian, C.M.; Cotton, S.L.; Paster, B.J.; Colombo, A.P.; Microbial signature profiles of periodontally healthy and diseased patients. J. Clin. Periodontol. 2014, 41, 1027–1036, 10.1111/jcpe.12302.
- Feres, M.; Teles, F.; Teles, R.; Figueiredo, L.C.; Faveri, M.; The subgingival periodontal microbiota of the aging mouth. Periodontology 2000 2016, 72, 30–53, 10.1111/prd.12136.
- Kawaguchi, H.; Hirachi, A.; Hasegawa, N.; Iwata, T.; Hamaguchi, H.; Shiba, H.; Takata, T.; Kato, Y.; Kurihara, H.; Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J. Periodontol. 2004, 75, 1281–1287, 10.1902/jop.2004.75.9.1281.
- Du, J.; Shan, Z.; Ma, P.; Wang, S.; Fan, Z.; Allogeneic bone marrow mesenchymal stem cell transplantation for periodontal regeneration.. J. Dent. Res. 2014, 93, 183–188, 10.1177/0022034513513026.
- Mohammed, E.; Khalil, E.; Sabry, D.; Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules 2018, 8, 167, 10.3390/biom8040167.
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S.; et al. Investigation of multipotent postnatal stem cells from human periodontal ligament.. Lancet 2004, 364, 149–155, 10.1016/S0140-6736(04)16627-0.
- Bartold, P.M.; Gronthos, S.; Ivanovski, S.; Fisher, A.; Hutmacher, D.W.; Tissue engineered periodontal products. J. Periodontal. Res. 2016, 51, 1–15, 10.1111/jre.12275.
- Iwata, T.; Yamato, M.; Zhang, Z.; Mukobata, S.; Washio, K.; Ando, T.; Feijen, J.; Okano, T.; Ishikawa, I; Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use.. J. Clin. Periodontol. 2010, 37, 1088–1099, 10.1111/j.1600-051X.2010.01597.x.
- Lim, J.C.; Bae, S.H.; Lee, G.; Ryu, C.J.; Jang, Y.J. Activation of beta-catenin by TGF-beta1 promotes ligament-fibroblastic differentiation and inhibits cementoblastic differentiation of human periodontal ligament cells. Stem Cells 2020. Online ahead of print.10.1002/stem.3275
- Bai, S.; Lee, J.H.; Son, C.; Lee, D.S.; Park, J.C; CPNE7 regenerates periodontal ligament via TAU-mediated alignment and cementum attachment protein-mediated attachment.. J. Clin. Periodontol. 2022, 49, 609–620, 10.1111/jcpe.13621.
- Park, C.H.; Oh, J.H.; Jung, H.M.; Choi, Y.; Rahman, S.U.; Kim, S.; Kim, T.I.; Shin, H.I.; Lee, Y.S.; Yu, F.H.; et al. Effects of the incorporation of epsilon-aminocaproic acid/chitosan particles to fibrin on cementoblast differentiation and cementum regeneration.. Acta Biomater. 2017, 61, 134–143, 10.1016/j.actbio.2017.07.039.
- Gauthier, R.; Jeannin, C.; Attik, N.; Trunfio-Sfarghiu, A.M.; Gritsch, K.; Grosgogeat, B.; Tissue Engineering for Periodontal Ligament Regeneration: Biomechanical Specifications.. J. Biomech. Eng. 2021, 143, 030801, 10.1115/1.4048810.
- Yang, L.; Yang, Y.; Wang, S.; Li, Y.; Zhao, Z.; In vitro mechanical loading models for periodontal ligament cells: From two-dimensional to three-dimensional models.. Arch. Oral Biol. 2015, 60, 416–424, 10.1016/j.archoralbio.2014.11.012.
- Natali, A.N.; Pavan, P.G.; Scarpa, C.; Numerical analysis of tooth mobility: Formulation of a non-linear constitutive law for the periodontal ligament. Dent. Mater 2004, 20, 623–629, 10.1016/j.dental.2003.08.003.
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G.; Cellular Mechanotransduction: From Tension to Function.. Front. Physiol. 2018, 9, 824, 10.3389/fphys.2018.00824.
- Schiller, H.B.; Fassler, R.; Mechanosensitivity and compositional dynamics of cell-matrix adhesions.. EMBO Rep 2013, 14, 509–519, 10.1038/embor.2013.49.
- Webster, K.D.; Ng, W.P.; Fletcher, D.A.; Tensional homeostasis in single fibroblasts.. Biophys. J. 2014, 107, 146–155, 10.1016/j.bpj.2014.04.051.
- Brown, R.A.; Prajapati, R.; McGrouther, D.A.; Yannas, I.V.; Eastwood, M.; Tensional homeostasis in dermal fibroblasts: Mechanical responses to mechanical loading in three-dimensional substrates.. J. Cell. Physiol 1998, 175, 323–332, 10.1002/(SICI)1097-4652(199806)175:3<323::AID-JCP10>3.0.CO;2-6.
- Kim, C.; Ye, F.; Ginsberg, M.H.; Regulation of integrin activation. Annu. Rev. Cell. Dev. Biol. 2011, 27, 321–345, 10.1146/annurev-cellbio-100109-104104.
- Calderwood, D.A.; Zent, R.; Grant, R.; Rees, D.J.; Hynes, R.O.; Ginsberg, M.H.; The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation.. J. Biol. Chem. 1999, 274, 28071–28074, 10.1074/jbc.274.40.28071.
- Haining, A.W.; von Essen, M.; Attwood, S.J.; Hytonen, V.P.; Del Rio Hernandez, A.; All Subdomains of the Talin Rod Are Mechanically Vulnerable and May Contribute to Cellular Mechanosensing.. ACS Nano 2016, 10, 6648–6658, 10.1021/acsnano.6b01658.
- Carisey, A.; Tsang, R.; Greiner, A.M.; Nijenhuis, N.; Heath, N.; Nazgiewicz, A.; Kemkemer, R.; Derby, B.; Spatz, J.; Ballestrem, C.; et al. Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner.. Curr. Biol. 2013, 23, 271–281, 10.1016/j.cub.2013.01.009.
- Kiema, T.; Lad, Y.; Jiang, P.; Oxley, C.L.; Baldassarre, M.; Wegener, K.L.; Campbell, I.D.; Ylanne, J.; Calderwood, D.A.; The molecular basis of filamin binding to integrins and competition with talin.. Mol. Cell 2006, 21, 337–347, 10.1016/j.molcel.2006.01.011.
- Das, M.; Ithychanda, S.S.; Qin, J.; Plow, E.F.; Migfilin and filamin as regulators of integrin activation in endothelial cells and neutrophils. PLoS ONE 2011, 6, e26355, 10.1371/journal.pone.0026355.
- Shifrin, Y.; Pinto, V.I.; Hassanali, A.; Arora, P.D.; McCulloch, C.A.; Force-induced apoptosis mediated by the Rac/Pak/p38 signalling pathway is regulated by filamin A.. Biochem. J. 2012, 445, 57–67, 10.1042/BJ20112119.
- Li, Y.; Zheng, W.; Liu, J.S.; Wang, J.; Yang, P.; Li, M.L.; Zhao, Z.H.; Expression of osteoclastogenesis inducers in a tissue model of periodontal ligament under compression. J. Dent. Res. 2011, 90, 115–120, 10.1177/0022034510385237.
- Li, Y.; Li, M.; Tan, L.; Huang, S.; Zhao, L.; Tang, T.; Liu, J.; Zhao, Z.; Analysis of time-course gene expression profiles of a periodontal ligament tissue model under compression.. Arch. Oral Biol. 2013, 58, 511–522, 10.1016/j.archoralbio.2012.10.006.
- Kang, K.L.; Lee, S.W.; Ahn, Y.S.; Kim, S.H.; Kang, Y.G.; Bioinformatic analysis of responsive genes in two-dimension and three-dimension cultured human periodontal ligament cells subjected to compressive stress. J. Periodontal Res. 2013, 48, 87–97, 10.1111/j.1600-0765.2012.01507.x.
- Feng, L.; Yang, R.; Liu, D.; Wang, X.; Song, Y.; Cao, H.; He, D.; Gan, Y.; Kou, X.; Zhou, Y.; et al. PDL Progenitor-Mediated PDL Recovery Contributes to Orthodontic Relapse. J. Dent. Res. 2016, 95, 1049–1056, 10.1177/0022034516648604.
- Jin, S.S.; He, D.Q.; Wang, Y.; Zhang, T.; Yu, H.J.; Li, Z.X.; Zhu, L.S.; Zhou, Y.H.; Liu, Y.; Mechanical force modulates periodontal ligament stem cell characteristics during bone remodelling via TRPV4.. Cell Prolif. 2020, 53, e12912, 10.1111/cpr.12912.
- Brockhaus, J.; Craveiro, R.B.; Azraq, I.; Niederau, C.; Schroder, S.K.; Weiskirchen, R.; Jankowski, J.; Wolf, M.; In Vitro Compression Model for Orthodontic Tooth Movement Modulates Human Periodontal Ligament Fibroblast Proliferation, Apoptosis and Cell Cycle. Biomolecules 2021, 11, 932, 10.3390/biom11070932.
- Stemmler, A.; Symmank, J.; Steinmetz, J.; von Brandenstein, K.; Hennig, C.L.; Jacobs, C.; GDF15 Supports the Inflammatory Response of PdL Fibroblasts Stimulated by P. gingivalis LPS and Concurrent Compression.. Int. J. Mol. Sci. 2021, 22, 13608, 10.3390/ijms222413608.
- Jiang, N.; He, D.; Ma, Y.; Su, J.; Wu, X.; Cui, S.; Li, Z.; Zhou, Y.; Yu, H.; Liu, Y.; et al. Force-Induced Autophagy in Periodontal Ligament Stem Cells Modulates M1 Macrophage Polarization via AKT Signaling. Front. Cell Dev. Biol. 2021, 9, 666631, 10.3389/fcell.2021.666631.
- Zhang, L.; Liu, W.; Zhao, J.; Ma, X.; Shen, L.; Zhang, Y.; Jin, F.; Jin, Y.; Mechanical stress regulates osteogenic differentiation and RANKL/OPG ratio in periodontal ligament stem cells by the Wnt/beta-catenin pathway. Biochim. Biophys. Acta 2016, 1860, 2211–2219, 10.1016/j.bbagen.2016.05.003.
- Yamamoto, T.; Kita, M.; Kimura, I.; Oseko, F.; Terauchi, R.; Takahashi, K.; Kubo, T.; Kanamura, N.; Mechanical stress induces expression of cytokines in human periodontal ligament cells.. Oral Dis. 2006, 12, 171–175, 10.1111/j.1601-0825.2005.01179.x.
- Wenger, K.H.; El-Awady, A.R.; Messer, R.L.; Sharawy, M.M.; White, G.; Lapp, C.A.; Pneumatic pressure bioreactor for cyclic hydrostatic stress application: Mechanobiology effects on periodontal ligament cells.. J. Appl. Physiol. (1985) 2011, 111, 1072–1079, 10.1152/japplphysiol.01175.2010.
- Yousefian, J.; Firouzian, F.; Shanfeld, J.; Ngan, P.; Lanese, R.; Davidovitch, Z.; A new experimental model for studying the response of periodontal ligament cells to hydrostatic pressure.. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 402–409, 10.1016/s0889-5406(95)70038-2.
- Jia, R.; Yi, Y.; Liu, J.; Pei, D.; Hu, B.; Hao, H.; Wu, L.; Wang, Z.; Luo, X.; Lu, Y.; et al. Cyclic compression emerged dual effects on the osteogenic and osteoclastic status of LPS-induced inflammatory human periodontal ligament cells according to loading force.. BMC Oral Health. 2020, 20, 7, 10.1186/s12903-019-0987-y.
- Saminathan, A.; Sriram, G.; Vinoth, J.K.; Cao, T.; Meikle, M.C.; Engineering the periodontal ligament in hyaluronan-gelatin-type I collagen constructs: Upregulation of apoptosis and alterations in gene expression by cyclic compressive strain.. Tissue Eng. Part A 2015, 21, 518–529, 10.1089/ten.TEA.2014.0221.
- Nettelhoff, L.; Grimm, S.; Jacobs, C.; Walter, C.; Pabst, A.M.; Goldschmitt, J.; Wehrbein, H.; Influence of mechanical compression on human periodontal ligament fibroblasts and osteoblasts. Clin. Oral Investig. 2016, 20, 621–629, 10.1007/s00784-015-1542-0.
- Saminathan, A.; Vinoth, K.J.; Low, H.H.; Cao, T.; Meikle, M.C.; Engineering three-dimensional constructs of the periodontal ligament in hyaluronan-gelatin hydrogel films and a mechanically active environment.. J. Periodontal Res. 2013, 48, 790–801, 10.1111/jre.12072.
- Chen, Y.; Mohammed, A.; Oubaidin, M.; Evans, C.A.; Zhou, X.; Luan, X.; Diekwisch, T.G.; Atsawasuwan, P.; Cyclic stretch and compression forces alter microRNA-29 expression of human periodontal ligament cells. Gene 2015, 566, 13–17, 10.1016/j.gene.2015.03.055.
- Wei, F.; Liu, D.; Feng, C.; Zhang, F.; Yang, S.; Hu, Y.; Ding, G.; Wang, S.; microRNA-21 mediates stretch-induced osteogenic differentiation in human periodontal ligament stem cells.. Stem Cells Dev. 2015, 24, 312–319, 10.1089/scd.2014.0191.
- Shen, T.; Qiu, L.; Chang, H.; Yang, Y.; Jian, C.; Xiong, J.; Zhou, J.; Dong, S.; Cyclic tension promotes osteogenic differentiation in human periodontal ligament stem cells.. Int. J. Clin. Exp. Pathol. 2014, 7, 7872–7880, PMC4270604.
- Wang, H.; Feng, C.; Jin, Y.; Tan, W.; Wei, F.; Identification and characterization of circular RNAs involved in mechanical force-induced periodontal ligament stem cells.. J. Cell. Physiol. 2019, 234, 10166–10177, 10.1002/jcp.27686.
- Xi, X.; Zhao, Y.; Liu, H.; Li, Z.; Chen, S.; Liu, D.; Nrf2 activation is involved in osteogenic differentiation of periodontal ligament stem cells under cyclic mechanical stretch.. Exp. Cell Res. 2021, 403, 112598, 10.1016/j.yexcr.2021.112598.
- Meng, X.; Wang, W.; Wang, X.; MicroRNA-34a and microRNA-146a target CELF3 and suppress the osteogenic differentiation of periodontal ligament stem cells under cyclic mechanical stretch. J. Dent. Sci. 2022, 17, 1281–1291, 10.1016/j.jds.2021.11.011.
- Liu, J.; Li, Q.; Liu, S.; Gao, J.; Qin, W.; Song, Y.; Jin, Z.; Periodontal Ligament Stem Cells in the Periodontitis Microenvironment Are Sensitive to Static Mechanical Strain.. Stem Cells Int. 2017, 2017, 1380851, 10.1155/2017/1380851.
- Salim, C.; Muders, H.; Jager, A.; Konermann, A.; Role of chaperone-assisted selective autophagy (CASA) in mechanical stress protection of periodontal ligament cells.. J. Orofac. Orthop. 2022, 83, 1–12, 10.1007/s00056-021-00358-3.
- Ulbricht, A.; Eppler, F.J.; Tapia, V.E.; van der Ven, P.F.; Hampe, N.; Hersch, N.; Vakeel, P.; Stadel, D.; Haas, A.; Saftig, P.; et al. Cellular mechanotransduction relies on tension-induced and chaperone-assisted autophagy. Curr. Biol. 2013, 23, 430–435, 10.1016/j.cub.2013.01.064.
- Ehrlicher, A.J.; Nakamura, F.; Hartwig, J.H.; Weitz, D.A.; Stossel, T.P.; Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A.. Nature 2011, 478, 260–263, 10.1038/nature10430.
- Huang, H.; Yang, R.; Zhou, Y.H.; Mechanobiology of Periodontal Ligament Stem Cells in Orthodontic Tooth Movement.. Stem Cells Int. 2018, 2018, 6531216, 10.1155/2018/6531216.
- Nemoto, T.; Kajiya, H.; Tsuzuki, T.; Takahashi, Y.; Okabe, K.; Differential induction of collagens by mechanical stress in human periodontal ligament cells. Arch. Oral Biol. 2010, 55, 981–987, 10.1016/j.archoralbio.2010.08.004.
- Oortgiesen, D.A.; Yu, N.; Bronckers, A.L.; Yang, F.; Walboomers, X.F.; Jansen, J.A.; A three-dimensional cell culture model to study the mechano-biological behavior in periodontal ligament regeneration.. Tissue Eng. Part C Methods 2012, 18, 81–89, 10.1089/ten.TEC.2011.0367.
- Papadopoulou, A.; Iliadi, A.; Eliades, T.; Kletsas, D.; Early responses of human periodontal ligament fibroblasts to cyclic and static mechanical stretching. Eur. J. Orthod 2017, 39, 258–263, 10.1093/ejo/cjw075.
- Yu, W.; Su, X.; LI, M.; Wan, W.; Li, A.; Zhou, H.; Xu, F; Three-dimensional mechanical microenvironment enhanced osteogenic activity of mesenchymal stem cells-derived exosomes. Chem. Eng. J. 2021, 417, 128040, 10.1016/j.cej.2020.128040.
- Howard, P.S.; Kucich, U.; Taliwal, R.; Korostoff, J.M.; Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J. Periodontal Res. 1998, 33, 500–508, 10.1111/j.1600-0765.1998.tb02350.x.
- Xu, C.; Fan, Z.; Shan, W.; Hao, Y.; Ma, J.; Huang, Q.; Zhang, F.; Cyclic stretch influenced expression of membrane connexin 43 in human periodontal ligament cell. Arch. Oral Biol. 2012, 57, 1602–1608, 10.1016/j.archoralbio.2012.07.002.
- Tang, N.; Zhao, Z.; Zhang, L.; Yu, Q.; Li, J.; Xu, Z.; Li, X.; Up-regulated osteogenic transcription factors during early response of human periodontal ligament stem cells to cyclic tensile strain. Arch. Med. Sci. 2012, 8, 422–430, 10.5114/aoms.2012.28810.
- Tang, M.; Peng, Z.; Mai, Z.; Chen, L.; Mao, Q.; Chen, Z.; Chen, Q.; Liu, L.; Wang, Y.; Ai, H.; et al. Fluid shear stress stimulates osteogenic differentiation of human periodontal ligament cells via the extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling pathways. J. Periodontol. 2014, 85, 1806–1813, 10.1902/jop.2014.140244.
- Zheng, L.; Chen, L.; Chen, Y.; Gui, J.; Li, Q.; Huang, Y.; Liu, M.; Jia, X.; Song, W.; Ji, J.; et al. The effects of fluid shear stress on proliferation and osteogenesis of human periodontal ligament cells. J. Biomech. 2016, 49, 572–579, 10.1016/j.jbiomech.2016.01.034.
- Shi, Q.; Zheng, L.; Na, J.; Li, X.; Yang, Z.; Chen, X.; Song, Y.; Li, C.; Zhou, L.; Fan, Y.; et al. Fluid shear stress promotes periodontal ligament cells proliferation via p38-AMOT-YAP. Cell. Mol. Life Sci. 2022, 79, 551, 10.1007/s00018-022-04591-w.
- Kim, S.G.; Kim, S.G.; Viechnicki, B.; Kim, S.; Nah, H.D.; Engineering of a periodontal ligament construct: Cell and fibre alignment induced by shear stress. J. Clin. Periodontol. 2011, 38, 1130–1136, 10.1111/j.1600-051X.2011.01790.x.
- Lin, H.H.; Chao, P.G.; Tai, W.C.; Chang, P.C.; 3D-Printed Collagen-Based Waveform Microfibrous Scaffold for Periodontal Ligament Reconstruction.. Int. J. Mol. Sci. 2021, 22, 7725, 10.3390/ijms22147725.
- Putame, G.; Gabetti, S.; Carbonaro, D.; Meglio, F.D.; Romano, V.; Sacco, A.M.; Belviso, I.; Serino, G.; Bignardi, C.; Morbiducci, U.; et al. Compact and tunable stretch bioreactor advancing tissue engineering implementation. Application to engineered cardiac constructs. Med. Eng. Phys. 2020, 84, 1–9, 10.1016/j.medengphy.2020.07.018.
- Lim, D.; Renteria, E.S.; Sime, D.S.; Ju, Y.M.; Kim, J.H.; Criswell, T.; Shupe, T.D.; Atala, A.; Marini, F.C.; Gurcan, M.N.; et al. Bioreactor design and validation for manufacturing strategies in tissue engineering. Bio-Des. Manuf. 2022, 5, 43–63, 10.1007/s42242-021-00154-3.
- Gabetti, S.; Masante, B.; Cochis, A.; Putame, G.; Sanginario, A.; Armando, I.; Fiume, E.; Scalia, A.C.; Daou, F.; Baino, F.; et al. An automated 3D-printed perfusion bioreactor combinable with pulsed electromagnetic field stimulators for bone tissue investigations. . Sci. Rep. 2022, 12, 13859, 10.1038/s41598-022-18075-1.
