Based on the issues listed above, it is clear that analyzing odor-active compounds is as important as it is tricky. A great effort was dedicated by the scientific community to develop and improve analytical procedures to make flavor analysis in compliance with the needs of the productive world: in the early years, when food analysis grew in its importance, the main task was to improve reliability and performance. In more recent times, after the analytical methods for most flavor compounds became satisfactory, the attention was shifted to make procedures fast, inexpensive, efficient, and green.
2. Derivatization of VOAs in Wine and Beer Analysis
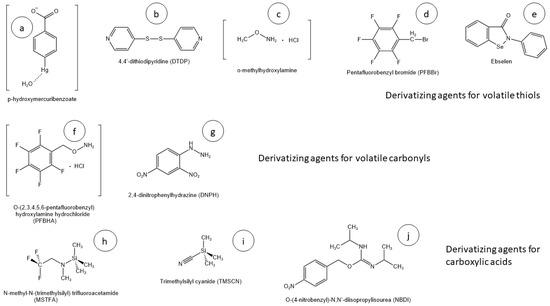
Most VOAs in wine and beer can be extracted and analyzed as they are, so derivatization concerns only a restricted pool of molecules. It must be highlighted that the need for derivatization is not only class-dependent but also structure-dependent. There are analytes belonging to the same group that could require or not require derivatization, depending on the olfactory threshold and volatility. To date, the most frequently derivatized VOAs are thiols, carboxylic acids, carbonyls, and some other extra compounds with particular characteristics. Early derivatization procedures involved transition metals or hazardous substances with consequent environmental and safety issues. The current approaches discussed below are based on organic or organometallic agents (Figure 1) with increased selectivity, yields, and reduced drawbacks.
Figure 1. (a–j) Chemical structures of most relevant derivatizing agents currently in use in VOAs’ analysis.
2.1. Volatile Thiols
Volatile Thiols (VTs), also known as mercaptans, are odor-active molecules functionalized with a R-SH functional group belonging to the broad category of Volatile Sulphur Compounds (VSCs). These compounds give a significant contribution to beverage aroma thanks to their broad presence and low Odor Detection Threshold (ODT) [
44]. Despite their important contribution, VTs are present in parts per trillion (ng·L
−1) levels, so an enrichment technique and a sensitive instrument are mandatory to perform analysis [
44,
45,
46]. In addition, due to the Sulphur reactivity, their concentration can be affected by several reactions and equilibria that take place in the matrix [
40,
47], which makes derivatization unavoidable. It must be underscored that extraction parameters which are labelled as huge for other green techniques must be reconsidered for VTs due to their peculiar characteristics.
Derivatizing methods had different focuses depending on the instrumental technique used. If the quantification was performed with GC, the aim was to increase volatility, whereas if it was performed with LC, the task was to increase the response of the detector. In all procedures the derivatizing agent reacted with the -SH group that, in free form, made the analyte highly reactive and unstable.
Historically, thiols were known to show a strong affinity for mercury (Hg+) and silver (Ag+), so first procedures were developed using these metal ions as highly selective derivatizing agents. Curiously, the word mercaptan itself derive from the Latin forms cercurium captans, which means mercury-seizing [
46]. Shifting to more recent times, traditional GC-based methods involve the use of metal ions or hazardous organomercurial agents like p-HMB (p-hydroxymercuribenzoate), require pH adjustment, large extraction volumes (over hundred mL), and are highly time-consuming (
Figure 1a) [
48]. Five hundred mL of wine are adjusted to pH 7 with sodium hydroxide and extracted 2 times with 100 mL of dichloromethane; the organic phase is then extracted with 20 mL of p-hydroxymercuribenzoate aqueous solution, keeping pH > 7. The resulting solution is finally purified and concentrated in a preparative column, eluted in dichloromethane again, and injected in GC-MS. The most relevant aspects of this and the following methods are reported in
Table 1. This procedure is highly time-consuming and requires huge volumes of sample and hazardous solvent, with consequent production of more than 1 L of waste per sample [
49]. However, this was the method which allowed the first instrumental studies on VTs’ occurrence in wine and, for over 10 years from its presentation, did not have any alternative [
50,
51,
52].
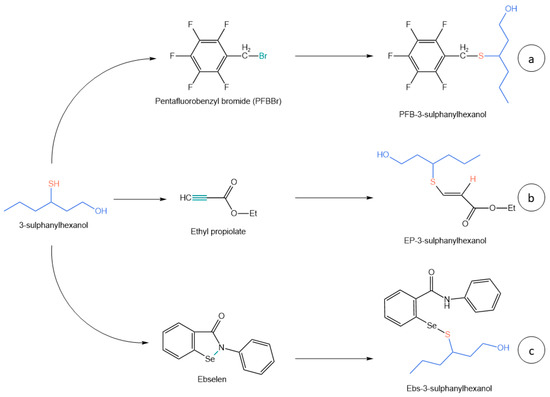
HS-SPME methods coupled to GC-MS-based techniques were interesting due to the high automation, avoided use of solvents, and the requirement of less than 20 mL of sample. Pentafluorobenzyl bromide (PFBBr) was successfully used as a derivatizing agent because, despite its toxicity, its selectivity and reaction efficiency require just a low amount of reagent, minimizing possible safety drawbacks (
Figure 1d) [
53]. PFBBr has a bromide atom bound to a benzylic primary carbon that perfectly matches with the requirements of nucleophilic substitution; in this case, the thiolic -SH acts as the nucleophile and replaces the Br leaving group, giving a more volatile and less polar product that better fits for gas chromatography (
Figure 2a) [
54].
Table 1. GC-MS and LC-MS methods presented for VTs’ determination and related highlights.
| Article |
Year |
Matrix |
Ext. Volume |
Ext. Technique |
Der. Agent |
Instrumentation |
Pro & Cons |
| [48] |
2003 |
White wine |
500 mL |
LLE + N2 concentration + preparative column |
p-HMB |
GC-EI-MS |
+ 5000 concentration factors
− 100 mL of hazardous solvent |
| [53] |
2006 |
White wine |
20 mL |
HS-SPME with on-fiber derivatization |
PFBBr |
GC-NCI-MS |
+ Solvent-free
− Time-consuming derivatizing process |
| [55] |
2007 |
White wine |
6 mL |
LLE with benzene |
PFBBr |
GC-NCI-MS |
+ No equipment required
− Time-consuming, hazardous solvent |
| [54] |
2008 |
White wine |
20 mL |
SPE and SIDA |
PFBBr |
GC-NCI-MS |
+ Good performance
− Disposable cartridge, use of solvents |
| [56] |
2014 |
White wine |
3 mL |
HS-SPME with in-situ derivatization |
o-methyl-hydroxylamine hydrochloride |
GC-EI-MS/MS |
+ Low LOD, high automation, low sample volume
− Only 4-MSP |
| [57] |
2015 |
Beer, hops, wort |
10 mL |
SBSE-PDMS with in-situ derivatization |
Ethyl propiolate |
GC-EI-MS/MS + GC-EI-QTof |
+ Low LODs, many analytes, solvent-free, safe reagents
− Instrumentation complexity |
| [58] |
2015 |
White wine |
20 mL |
SPE with Bond-Elut C18, and SIDA |
DTDP |
LC-MS/MS |
+ Relevant VTs, accuracy
− Disposable cartridge |
| [59] |
2018 |
Wine (all) |
20 mL |
SPE with Bond-Elut C18, and SIDA |
DTDP |
LC-HRMS |
+ Enantiomer analysis
− Disposable cartridge |
| [60] |
2018 |
Red wine |
20 mL |
SPE with Supelclean ENVI-18 |
DTDP |
GC-MS/MS |
+ Greener chromatography
− Disposable cartridge, complexity |
| [61] |
2015 |
Wine, beer |
20 mL |
LLE with 4 mL of CH2Cl2 |
Ebselen |
LC-HRMS |
+ No equipment required, flexibility, performance
− CH2Cl2, time-consuming |
| [62] |
2018 |
White wine |
35 mL |
LLE with ethanol |
Ebselen |
LC-HRMS |
+ No equipment required, safe solvent
− high sample volume, filtration |
| [63] |
2017 |
White wine |
100 mL |
SPE, 20 mg Li-Chrolut EN |
Ebselen |
LC-HRMS |
+ Minimized cartridge, accuracy
− High sample volume |
| [39] |
2022 |
White wine |
35 mL |
Micro LLE + 0.22 µm filtration |
Ebselen |
LC-MS/MS |
+ Performance, reduced volumes
− Low automatability |
Figure 2. Different derivatization pathways in VTs’ derivatization. (a) Reaction with PFBBr; (b) Reaction with ethyl propiolate; (c) Reaction with ebselen.
Thanks to its promising results, the same derivatizing agent was also used in a miniaturized LLE protocol [
55] and a miniaturized SPE one [
54]. HS-SPME was also used with direct in situ derivatization using o-methylhydroxylamine hydrochloride and stable isotope dilution assay [
56], obtaining an impressive LOD of 0.19 ng·L
−1 for 4-MSP in white wine. In this case, only 4-MSP was evaluated, because the o-methylhydroxylamine reacted with the carbonyl group instead of the -SH group (
Figure 1c).
Ethyl propiolate (ETP) was another interesting derivatizing agent used for thiol GC analysis. His reactant was a greener alternative to the most used PFBBr based on a different chemical mechanism. In this case, the thiolic analyte was added in alkaline pH (>10) to the triple C-C bond of ETP with an anti-Markonikov regioselectivity (
Figure 2b). Stir bar sorptive extraction (SBSE), which was an emerging green technique, was used coupled to in situ ETP derivatization and thermal desorption GC-MS/MS; that was one of the greenest methods developed up to now for thiols that covers a broad range of analytes with proper sensitivity [
57]. However, in the last decade, the focus has moved to preparation techniques suitable for coupling with LC-MS, which granted boosted sensitivity, simplifying the analyte isolation. Derivatization with 4,4′-dithiodipyridine (DTDP) takes place at mild acid conditions providing stable non-volatile molecules which are suitable for SPE isolation with a C18 extracting phase [
58]. DTDP reacted directly with -SH, producing an organosulfur molecule with a pyridinic site used to enhance ionization efficiency for ESI (
Figure 1b). With a similar procedure, it is also possible to isolate enantiomers in a different type of wine, which are known to provide different nuances, just by using an Amylose-1 chiral column [
59]. The SPE method described above was the progenitor of many other procedures: in the “greenest” one, the conventional LC was replaced by convergence chromatography (CC), which is a type of supercritical fluid chromatography (SFC) where CO
2 and methanol are used as a mobile phase [
60].
Even though DTDP and other derivatizing agents have been successfully used for VTs’ analysis, 2-phenyl-1,2-benzisoselenazol-3-one (ebselen) is the one which showed the best selectivity, efficiency, versatility, and stability. Ebselen reacts with thiols in acidic medium, simultaneously protecting the -SH function from oxidation and increasing the affinity of the derivatized molecule for the extraction solvent by the formation of a positive charge in the nitrogen atom of the derivatizing agent (
Figure 1e). The reaction mechanism (
Figure 2c) is based on the Se-N bond cleavage of ebselen by the thiolic function and the following formation of the corresponding selenenyl sulfide Se S bond [
64]. The exchangeable hydrogen of the amidic function contributes to the enhanced ionization efficiency in ESI sources, resulting in an improvement of the method response [
65].
Vichi et al. first published a method for olive oil [
66] that was subsequently also tailored for brewed coffee [
67,
68], beer, and wine [
61]. These protocols did not involve disposable consumables but require the use of small amounts of dichloromethane, which is not a green solvent. A similar procedure was presented for wines replacing dichloromethane with ethanol, but with a higher volume of sample (35 mL instead of 20 mL) [
62]; this method was further optimized, achieving LODs and LOQs suitable for the analysis of VTs also in non-varietal wines [
39].
2.2. Volatile Carbonyls
Volatile carbonyl compounds (VCCs) are fundamental components in the flavor of all fermented beverages. Because of their low odor perception threshold, these molecules are responsible for a strong olfactory impact even at low concentrations [
69,
70]. VCCs, both aldehydes and ketones, originate as products of Maillard reactions, Strecker degradation, aldol condensation, and lipid oxidation [
71] but also from biological processes like alcoholic fermentation. Because of that, these molecules are among the most relevant VOAs in fermented beverages [
33,
72]. A content of VCCs slightly above the olfactory threshold is related to aromatic and pleasant nuances of vanilla, caramel, butter, honey, potato, orange, lemon, violets, cider, and plum [
19,
73,
74,
75,
76,
77,
78,
79,
80]. Conversely, higher concentrations are associated with oxidation, which is a long-standing undesired problem responsible for aroma defects [
81,
82,
83].
From the analytical point of view, VCCs’ quantification is affected by two main issues. First, thanks to the presence of a functional group suitable for receiving the hydrogen bond, these molecules are among the most hydrophilic VOAs [
83]. In addition, the average concentration in principally fermented beverages is comprised between hundreds of ng·L
−1 and a few µg·L
−1, so the amount in the vapors is significantly low [
19].
Most current methods were based on heterogeneous extraction (SPE or SPME) and GC-MS quantification [
20]. For what concerns SPE, Mayr et al. developed a GC-MS/MS quantitation method for 18 carbonyl compounds based on O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine hydrochloride (PFBHA) derivatization on cartridge [
84]. PFBHA is an efficient and selective agent that reacts with carbonyl function through a nucleophile addiction, giving an oxime-like product (
Figure 1f) [
41]. Even though this method showed high performance in terms of sensitivity and linearity, the SPE procedure was expensive, time-consuming, and scarcely automatable, in contrast to the rules of green analytical chemistry [
85]. To overcome these limits, many other methods were based on the Head Space Solid Phase Micro Extraction technique (HS-SPME). This straightforward strategy does not involve any preliminary manual operation and combines high productivity and satisfactory performance [
86]. HS-SPME methods were purposed with PFBHA on-fiber derivatization [
87] and in-solution derivatization [
88,
89], both with satisfactory results but different simplicity of execution. Similar methods were also used to perform carbonyl quantitation in beers [
68].
On-fiber derivatization (OFD) was applied for the determination of staling 15 aldehydes in wort and beer samples using PFBHA and GC-EI-MS/MS [
90]. This procedure demonstrated an improved sensitivity over a broad calibration range (0.01–1000 µg·L
−1) and reduced matrix effects resulting from overlapping PFBHA-oximes (PFBOs). Extensive validation through linearity assessment (R
2 > 0.99), LOD/LOQ, precision (RSD < 9.2%), and recovery (80–118%) was provided to support the protocol. The procedure is very simple; 3 mL of decarbonized beer, 1 g NaCl, and 10 min at 50 °C of fiber exposure previously soaked with the derivatizing agent. A preliminary version of this method was presented some years before by Schmarr et al. for the determination of many VCCs in wine; in this case, a wider range of analytes was analyzed comprising alkanals, €-2-alkenals, (E,E)-2,4-alkadienals, and others, including S-containing ketones [
87]. This procedure required 10 mL of untreated sample and 20 min of following head-space extraction at 40 °C.
2.3. Carboxylic Acids
Carboxylic acids (or fatty acids) are hydrocarbons functionalized with a carboxyl group whose presence in fermented beverages originates from raw materials (mostly from the firm tissues of fruits) and, especially, during alcoholic fermentation [
94]. Due to the strong hydrophilic interactions established by the carboxyl group with the matrix, most of them are non-volatile and odorless [
95]. Despite that, some short-chain carboxylic acids are volatile enough to move into the vapors and to cause olfactory activity. As it happens for many VOAs, carboxylic acids are identified with desired flavors for some products like sour beers [
96], whereas with a higher concentration, they are related to unpleasant acrid and repulsive nuances [
97].
Conventional fatty acid quantitation was performed with an extraction followed by derivatization to methyl esters and GC-MS analysis [
98]; in this case, the extraction was performed using methanol, which also had the function of a derivatizing agent for thorough esterification in acid conditions [
99]. This method, which was developed over 30 years ago and is still in use, was affected by the simultaneous transesterification between methanol and ethyl esters present in the samples; this issue determined an increased amount of free fatty acids and a non-representative measurement of other VOAs. Gallart et al. presented an alternative procedure based on methylation for a precise quantitation of free fatty acids, spanning from C6 to C18 [
100]; these are key compounds for wines and beers, since C6 (caproic acid), C8 (caprylic acid), and C10 (capric acid) are important VOAs because of their flavors of rancid cheese and goat-like flavors, which are unpleasant already at a high concentration. In this upgraded protocol, the extraction was performed in triplicate using hexane (5 mL), the sum of aliquots was then centrifuged and concentrated to 1 mL under a nitrogen stream, and finally 1 mL of derivatizing solution (sulfuric acid (3%) in methanol) was injected and allowed to react for 3 h at room temperature. This procedure was more complex but made the quantitation of free fatty acids feasible and precise.
In more recent times, silylation of the carboxyl function was implemented as a selective derivatization strategy for the analysis of free carboxylic acids. This procedure was used in many other matrices before and only in the last ten years was extended to beverages [
101]. Silylation proceeds via bimolecular nucleophilic substitution (SN2) on the silicon atom (electrophile) where the carboxyl group acts as the nucleophile that replaces a part of the derivatizing reagent (leaving group). The aim of this process is to hide the hydrophilic carboxyl function and to simultaneously lower the polarity and increase the volatility [
102]. Browsing published literature within all developed reactants for silylation purposes, only two of them were used in beverages. Zhang et al. optimized a method based on the use of [N-methyl-N-(trimethylsilyl) trifluoroacetamide] MSTFA, the application field of which was the metabolomics of plant leaves (
Figure 1h) [
103]. The authors implemented a lyophilization (10 h at room temperature) prior to sample drying, dissolution in methoxyamine hydrochloride (20 mg·L
−1 in pyridine), silylation with MSTFA for 30 min at 37 °C, and a final filtration. This method demonstrated a significant efficiency and good versatility (amines and monosaccharides were also simultaneously derivatized).