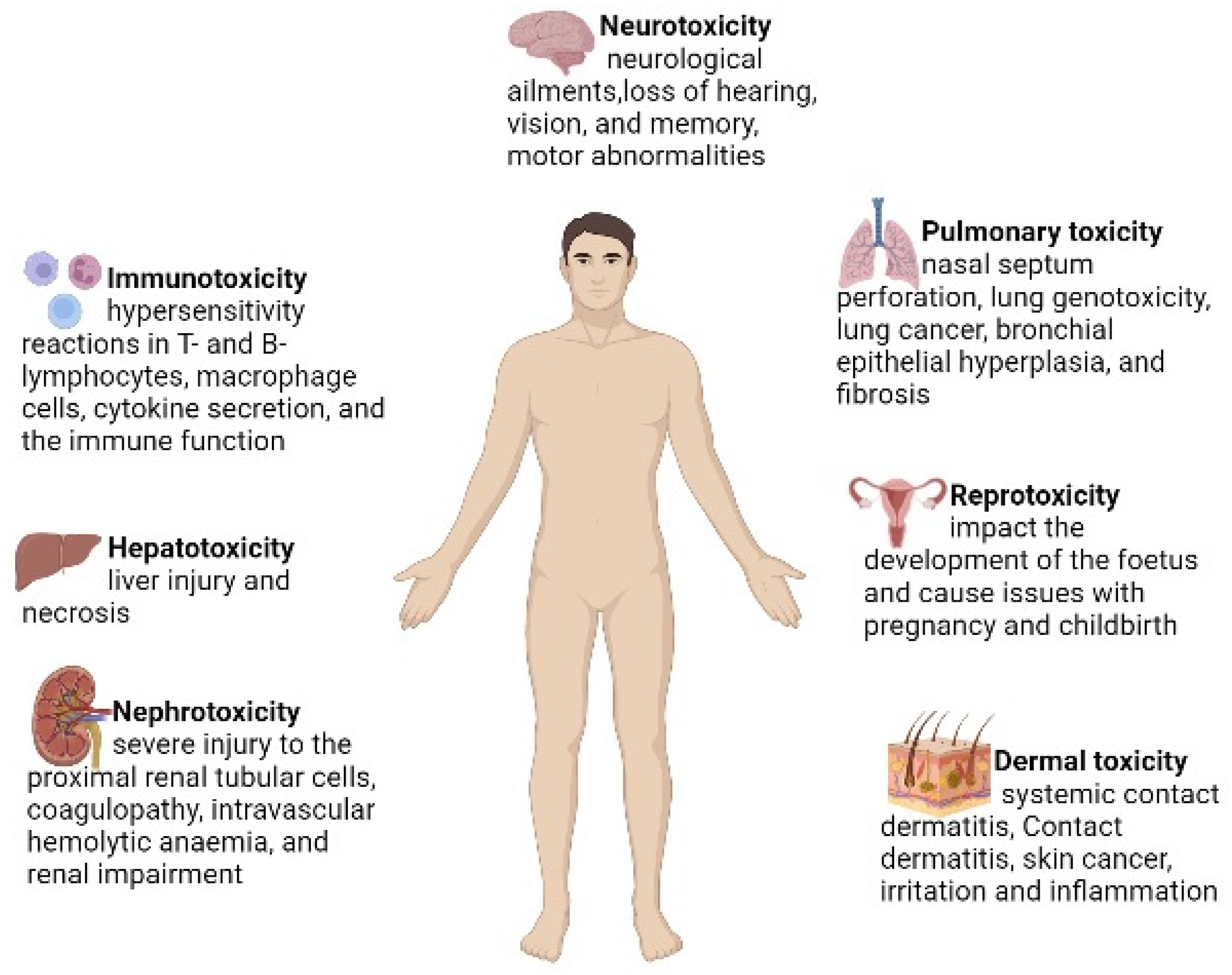
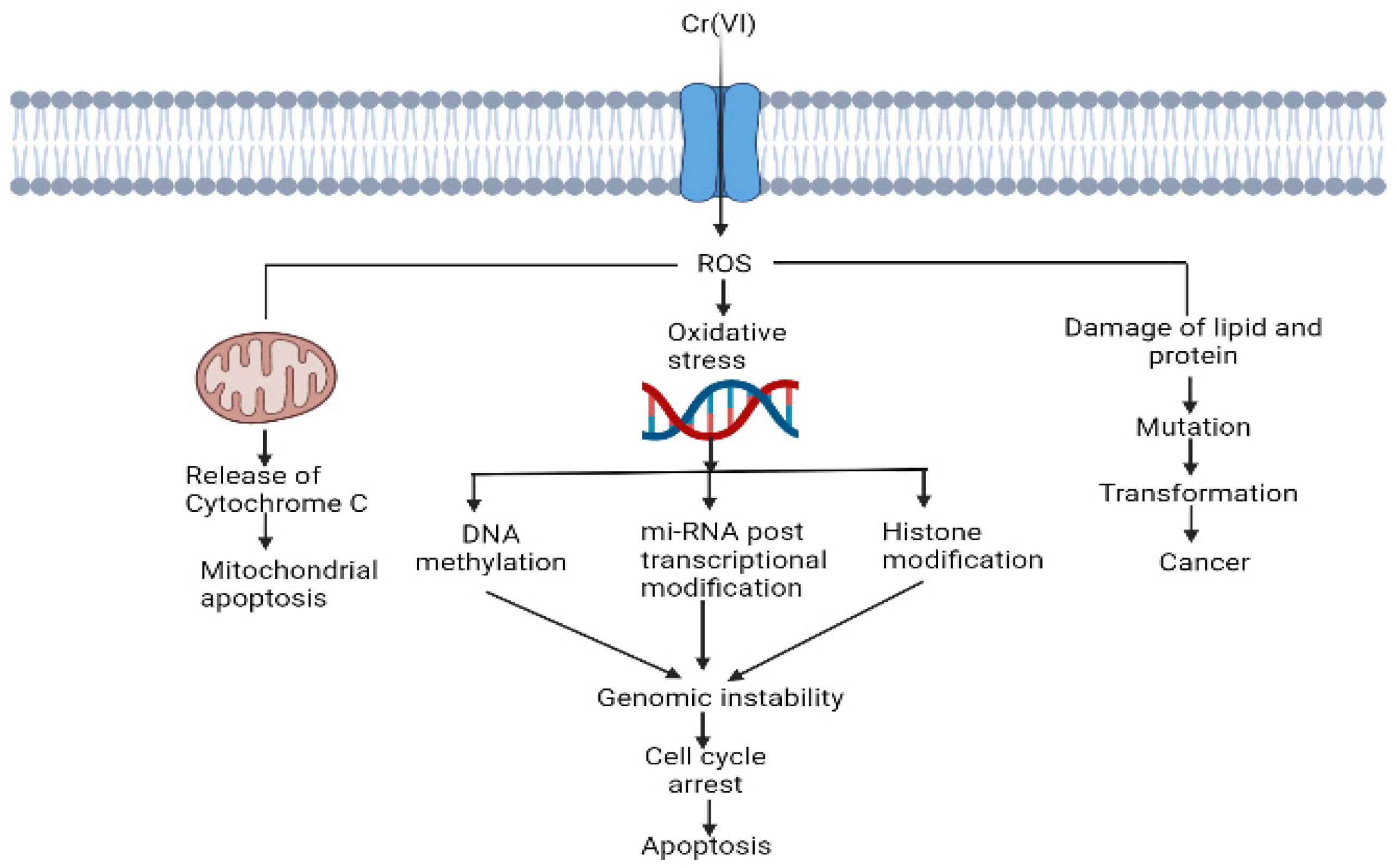
Hexavalent chromium is a highly soluble environmental contaminant. It is a widespread anthropogenic chromium species that is 100 times more toxic than trivalent chromium. Leather, chrome plating, coal mining and paint industries are the major sources of hexavalent chromium in water. Hexavalent chromium is widely recognised as a carcinogen and mutagen in humans and other animals. It is also responsible for multiorgan damage, such as kidney damage, liver failure, heart failure, skin disease and lung dysfunction. The fate of the toxicity of hexavalent chromium depends on its oxidation state. The reduction of Cr (VI) to Cr (III) is responsible for the generation of reactive oxygen species (ROS) and chromium intermediate species, such as Cr (V) and Cr (IV). Reactive oxygen species (ROS) are responsible for oxidative tissue damage and the disruption of cell organelles, such as mitochondria, DNA, RNA and protein molecules. Cr (VI)-induced oxidative stress can be neutralised by the antioxidant system in human and animal cells.
- hexavalent chromium
- source of Cr (VI)
- Cr (VI) toxicity
- reactive oxygen species
- antioxidants
1. Introduction
2. Cr (VI)-Induced Oxidative Stress and Disruption of Cell Components
| Cell Type | Impact | Citation |
|---|---|---|
| Cardiomyocytes of broiler | Apoptosis-related genes Bax and p53 expression, mitochondrial malfunction and oxidative stress, myocardial apoptosis and autophagy | [33] |
| Liver cell of zebrafish | Downregulation of the Bcl2 gene and transcriptional activation of apoptosis-related p53, Bax, caspase 9 and caspase 3 genes, which changes the elemental composition of the liver | [34] |
| Liver and kidney cells of Sprague-Dawley rats | Dose- and time-dependent effects induced DNA damage due to increase in ROS levels | [35] |
| Hepatocytes (HepG2) of humans | Mitochondrial damage, apoptosis, oxidative stress |
[36] |
| Lung epithelial cells of rats | ROS-induced cell death and activation of the p53-related pathway | [37] |
| Liver and kidney cells of carassius auratus | Oxidative stress, genotoxicity and histopathology | [38] |
| Bronchial epithelial cells (Beas-2B) of humans | Cell transformation | [39] |
| Liver and brain tissues of mice | Oxidative stress and tissue damage | [40] |
| Gill and kidney cells of Anguilla anguilla L. | Genotoxicity at a higher concentration in gills and at all concentrations in kidneys | [41] |
| Kidney cells of Wistar rats | Apoptosis and autophagy, oxidative stress in kidneys, mitochondrial dynamics disorder via inhibiting the sirt/pgc-1a pathway | [42] |
3. Antioxidants and Their Protective Role against Cr (VI) Toxicity
3.1. Enzymatic Antioxidants
3.1.1. Superoxide Dismutase (EC 1.15.1.1)
3.2. Endogenous and Exogenous Antioxidants
3.3. Polyphenolic Antioxidants
This entry is adapted from the peer-reviewed paper 10.3390/antiox11122375
References
- Wong, M.H. Environmental Contamination: Health Risks and Ecological Restoration; CRC Press: Boca Raton, FL, USA, 2012.
- Fergusson, J.E. The Heavy Elements: Chemistry, Environmental Impact and Health Effects; Pergamon Press: Oxford, UK, 1990; pp. 85–547.
- Wu, M.; Xu, Y.; Ding, W.; Li, Y.; Xu, H. Mycoremediation of manganese and phenanthrene by Pleurotus eryngii mycelium enhanced by Tween 80 and saponin. Appl. Microbiol. Biotechnol. 2016, 100, 7249–7261.
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691.
- Flora, S.J.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788.
- Sobhanardakani, S.; Zandi Pak, R.; Mohammadi, M.J. Removal of Ni (II) and Zn (II) from aqueous solutions using chitosan. Arch. Hyg. Sci. 2016, 5, 47–55.
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377.
- Rai, P.K. Heavy metal pollution in aquatic ecosystems and its phytoremediation using wetland plants: An ecosustainable approach. Int. J. Phytorem. 2008, 10, 131–158.
- Ghose, M.K. Design of cost-effective coal washery effluent treatment plant for clean environment. J. Sci. Ind. Res. 2001, 60, 40–47.
- ATSDR; Public Health Service. Toxicological Profile for Lead; ATSDR: Atlanta, GA, USA, 2020. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf (accessed on 1 March 2020).
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of hexavalent chromium-contaminated water and wastewater: A review. Water Air Soil Pollut. 2009, 200, 59–70.
- Central Pollution Control Board (CPCB). India (2000) Environmental Standards for Ambient Air, Automobiles, Fuels, Industries and Noise. Available online: http://www.cpcbenvis.nic.in/scanned%20reports/PCL%204%20Environmental%20Standards.pdf (accessed on 15 March 2018).
- Peterson-Roth, E.; Reynolds, M.; Quievryn, G.; Zhitkovich, A. Mismatch repair proteins are activators of toxic responses to chromium-DNA damage. Mol. Cell. Biol. 2005, 25, 3596–3607.
- Singh, V.; Singh, M.P.; Mishra, V. Bioremediation of toxic metal ions from coal washery effluent. Desalin. Water Treat. 2020, 197, 300–318.
- Singh, V.; Singh, J.; Mishra, V. Development of a cost-effective, recyclable and viable metal ion doped adsorbent for simultaneous adsorption and reduction of toxic Cr (VI) ions. J. Environ. Chem. Eng. 2021, 9, 105124–105137.
- Ahemad, M. Enhancing phytoremediation of chromium-stressed soils through plant-growth-promoting bacteria. J. Genet. Eng. Biotechnol. 2015, 13, 51–58.
- Kubrak, O.I.; Lushchak, V.; Lushchak, J.V.; Torous, I.M.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Chromium effects on free radical processes in goldfish tissues: Comparison of Cr (III) and Cr (VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 360–370.
- Kumar, M.S.; Praveenkumar, R.; Ilavarasi, A.; Rajeshwari, K.; Thajuddin, N. Biochemical changes of fresh water cyanobacteria Dolichospermum flos-aquae NTMS07 to chromium-induced stress with special reference to antioxidant enzymes and cellular fatty acids. Bull. Environ. Contam. Toxicol. 2013, 90, 730–735.
- Zafar, S.; Aqil, F.; Ahmad, I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour. Technol. 2007, 98, 2557–2561.
- Joutey, N.T.; Sayel, H.; Bahafid, W.; Ghachtouli, N. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev. Environ. Contam. Toxicol. 2015, 233, 45–69.
- Bokare, A.D.; Choi, W. Advanced oxidation process based on the Cr (III)/Cr (VI) redox cycle. Environ. Sci. Technol. 2011, 45, 9332–9338.
- Mishra, S.; Bharagava, R.N. Toxic and Genotoxic Effects of Hexavalent Chromium in Environment and Its Bioremediation Strategies. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 1–32.
- Li, X.; Abdel-Moneim, A.-M.E.; Yang, B. Signaling Pathways and Genes Associated with Hexavalent Chromium-Induced Hepatotoxicity. Biol. Trace Elem. Res. 2011, 225, 1–17.
- Chakraborty, R.; Renu, K.; Eladl, M.A.; El-Sherbiny, M.; Elsherbini, D.M.A.; Mirza, A.K.; Vellingiri, B.; Iyer, M.; Dey, A.; Gopalakrishnan, A.V. Mechanism of Chromium-Induced Toxicity in Lungs, Liver, and Kidney and Their Ameliorative Agents. Biomed. Pharmacother. 2022, 151, 113119.
- Kim, H.G.; Yoon, D.H.; Lee, W.H.; Han, S.K.; Shrestha, B.; Kim, C.H.; Lim, M.H.; Chang, W.; Lim, S.; Choi, S.; et al. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J. Ethnopharmacol. 2007, 114, 307–315.
- Bagchi, D.; Vuchetich, P.J.; Bagchi, M.; Hassoun, E.A.; Tran, M.X.; Tang, L.; Stohs, S.J. Induction of oxidative stress by chronic administration of sodium dichromate and cadmium chloride to rats. Free Radical Biol. Med. 1997, 22, 471–478.
- Wise, S.S.; Holmes, A.L.; Ketterer, M.E.; Hartsock, W.J.; Fomchenko, E.; Katsifis, S.; Thompson, W.D.; Wise, J.P., Sr. Chromium is the proximate clastogenic species for lead chromate-induced clastogenicity in human bronchial cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2004, 560, 79–89.
- Wakeel, A.; Xu, M.; Gan, Y. Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int. J. Mol. Sci. 2020, 21, 728.
- Cohen, M.D.; Kargacin, B.; Klein, C.B.; Costa, M. Mechanisms of chromium carcinogenicity and toxicity. Crit. Rev. Toxicol. 1993, 23, 255–281.
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972.
- Mohamed, A.A.; El-Houseiny, W.; El-Murr, A.E.; Ebraheim, L.L.M.; Ahmed, A.I.; El-Hakim, Y.M.A. Effect of Hexavalent Chromium Exposure on the Liver and Kidney Tissues Related to the Expression of CYP450 and GST Genes of Oreochromis Niloticus Fish: Role of Curcumin Supplemented Diet. Ecotoxicol. Environ. Saf. 2020, 188, 109890.
- Li, S.; Baiyun, R.; Lv, Z.; Li, J.; Han, D.; Zhao, W.; Yu, L.; Deng, N.; Liu, Z.; Zhang, Z. Exploring the Kidney Hazard of Exposure to Mercuric Chloride in Mice: Disorder of Mitochondrial Dynamics Induces Oxidative Stress and Results in Apoptosis. Chemosphere 2019, 234, 822–829.
- Li, H.; Shi, J.; Gao, H.; Yang, X.; Fu, Y.; Peng, Y.; Xia, Y.; Zhou, D. Hexavalent Chromium Causes Apoptosis and Autophagy by Inducing Mitochondrial Dysfunction and Oxidative Stress in Broiler Cardiomyocytes. Biol. Trace Elem. Res. 2022, 200, 2866–2875.
- Shaw, P.; Mondal, P.; Dey Bhowmik, A.; Bandyopadhyay, A.; Sudarshan, M.; Chakraborty, A.; Chattopadhyay, A. Environmentally Relevant Hexavalent Chromium Disrupts Elemental Homeostasis and Induces Apoptosis in Zebrafish Liver. Bull. Environ. Contam. Toxicol. 2022, 108, 716–724.
- Patlolla, A.K.; Barnes, C.; Yedjou, C.; Velma, V.R.; Tchounwou, P.B. Oxidative Stress, DNA Damage, and Antioxidant Enzyme Activity Induced by Hexavalent Chromium in Sprague-Dawley Rats. Environ Toxicol. 2009, 24, 66–73.
- Das, J.; Sarkar, A.; Sil, P.C. Hexavalent Chromium Induces Apoptosis in Human Liver (HepG2) Cells via Redox Imbalance. Toxicol. Rep. 2015, 2, 600–608.
- Lei, T.; He, Q.Y.; Cai, Z.; Zhou, Y.; Wang, Y.L.; Si, L.S.; Cai, Z.; Chiu, J.F. Proteomic Analysis of Chromium Cytotoxicity in Cultured Rat Lung Epithelial Cells. Proteomics 2008, 8, 2420–2429.
- Velma, V.; Tchounwou, P.B. Chromium-Induced Biochemical, Genotoxic and Histopathologic Effects in Liver and Kidney of Goldfish, Carassius Auratus. Mutat. Res. 2010, 698, 43–51.
- Wang, X.; Son, Y.O.; Chang, Q.; Sun, L.; Hitron, J.A.; Budhraja, A.; Zhang, Z.; Ke, Z.; Chen, F.; Luo, J.; et al. NADPH Oxidase Activation Is Required in Reactive Oxygen Species Generation and Cell Transformation Induced by Hexavalent Chromium. Toxicol. Sci. 2011, 123, 399–410.
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and Oxidative Mechanisms of Different Forms of Chromium. Toxicology 2002, 180, 5–22.
- Ahmad, I.; Maria, V.L.; Oliveira, M.; Pacheco, M.; Santos, M.A. Oxidative stress and genotoxic effects in gill and kidney of Anguilla anguilla L. exposed to chromium with or without pre-exposure to beta-naphthoflavone. Mutat. Res. 2006, 608, 16–28.
- Zheng, X.; Li, S.; Li, J.; Lv, Y.; Wang, X.; Wu, P.; Yang, Q.; Tang, Y.; Liu, Y.; Zhang, Z. Hexavalent Chromium Induces Renal Apoptosis and Autophagy via Disordering the Balance of Mitochondrial Dynamics in Rats. Ecotoxicol. Environ. Saf. 2020, 204, 111061.
- Xu, J.; Zhao, M.; Pei, L.; Zhang, R.; Liu, X.; Wei, L.; Yang, M.; Xu, Q. Oxidative stress and DNA damage in a long-term hexavalent chromium-exposed population in North China: A cross-sectional study. BMJ Open 2018, 8, e021470.
- Pavesi, T.; Moreira, J.C. Mechanisms and Individuality in Chromium Toxicity in Humans. J. Appl. Toxicol. 2020, 40, 1183–1197.
- Bagchi, D.; Bagchi, M.; Stohs, S.J. Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Mol. Cell. Biochem. 2001, 222, 149–158.
- Holmes, A.L.; Wise, S.S.; Wise, J.P., Sr. Carcinogenicity of hexavalent chromium. Indian J. Med. Res. 2008, 128, 353–372.
- Son, Y.O.; Hitron, J.A.; Wang, X.; Chang, Q.; Pan, J.; Zhang, Z.; Liu, J.; Wang, S.; Lee, J.C.; Shi, X. Cr(VI) induces mitochondrial-mediated and caspase-dependent apoptosis through reactive oxygen species-mediated p53 activation in JB6 Cl41 cells. Toxicol. Appl. Pharmacol. 2010, 245, 226–235.
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent Developments in Essentiality of Trivalent Chromium and Toxicity of Hexavalent Chromium: Implications on Human Health and Remediation Strategies. J. Hazard. Mater. Adv. 2022, 7, 100113.
- Dworzański, J.; Strycharz-Dudziak, M.; Kliszczewska, E.; Kiełczykowska, M.; Dworzańska, A.; Drop, B.; Polz-Dacewicz, M. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. PLoS ONE 2020, 15, e0230374.
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 7282–7300.
- Azadmanesh, J.; Borgstahl, G.E.O. A review of the catalytic mechanism of human manganese superoxide dismutase. Antioxidants 2018, 7, 25.
- Lewandowski, L.; Kepinska, M.; Milnerowicz, H. The copper-zinc superoxide dismutase activity in selected diseases. Eur. J. Clin. Investig. 2019, 49, e13036.
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous Non-Enzymatic Antioxidants in the Human Body. Adv. Med. Sci. 2018, 63, 68–78.
- Bouayed, J.; Bohn, T. Exogenous Antioxidants—Double-Edged Swords in Cellular Redox State: Health Beneficial Effects at Physiologic Doses versus Deleterious Effects at High Doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237.
- Husain, N.; Mahmood, R. 3,4-Dihydroxybenzaldehyde Quenches ROS and RNS and Protects Human Blood Cells from Cr(VI)-Induced Cytotoxicity and Genotoxicity. Toxicol. Vitr. 2018, 50, 293–304.
- Jeong, J.B.; Chul Hong, S.; Jin Jeong, H. 3,4-Dihydroxybenzaldehyde Purified from the Barley Seeds (Hordeum vulgare) Inhibits Oxidative DNA Damage and Apoptosis via Its Antioxidant Activity. Phytomedicine 2009, 16, 85–94.
- Gao, J.W.; Yamane, T.; Maita, H.; Ishikawa, S.; Iguchi-Ariga, S.M.M.; Pu, X.P.; Ariga, H. DJ-1-Mediated Protective Effect of Protocatechuic Aldehyde against Oxidative Stress in SH-SY5Y Cells. J. Pharmacol. Sci. 2011, 115, 36–44.
- Cuevas-Magaña, M.Y.; Vega-García, C.C.; León-Contreras, J.C.; Hernández-Pando, R.; Zazueta, C.; García-Niño, W.R. Ellagic Acid Ameliorates Hexavalent Chromium-Induced Renal Toxicity by Attenuating Oxidative Stress, Suppressing TNF-α and Protecting Mitochondria. Toxicol. Appl. Pharmacol. 2022, 454, 116242.
- Losso, J.N.; Bansode, R.R.; Trappey, A.; Bawadi, H.A.; Truax, R. In Vitro Anti-Proliferative Activities of Ellagic Acid. J. Nutr. Biochem. 2004, 15, 672–678.
- Li, R.; Fan, X.; Zhang, T.; Song, H.; Bian, X.; Nai, R.; Li, J.; Zhang, J. Expression of selenium-independent glutathione peroxidase 5 (GPx5) in the epididymis of Small Tail Han sheep. Asian-Australas. J. Anim. Sci. 2018, 31, 1591–1597.
