Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Intranasal administration has gained an increasing interest for brain drug delivery since it allows direct transport through neuronal pathways, which can be quite advantageous for central nervous system disorders, such as depression and anxiety. Nanoparticles have been studied as possible alternatives to conventional formulations, with the objective of improving drug bioavailability.
- anxiety
- depression
- intranasal
- nose-to-brain
- nanoparticles
1. Intranasal Administration for Brain Drug Delivery
The intranasal route of administration is an alternative to the administration of drugs in the treatment of CNS pathologies which has several advantages over other routes, allowing to overcome the blood-brain barrier; transport of molecules of larger dimensions (up to about 1000 Da); avoid the first pass hepatic metabolism; minimize the side effects caused by drugs administered through the systemic circulation, reducing toxicity; reduce the drug dose that is necessary to achieve a therapeutically effective concentration at the site of action, and thus reach the therapeutic threshold. Additionally, in general, as a route of administration, it is simple, practical, and convenient since it does not require administration techniques involving coordination or swallowing (such as the oral route) or the aid of a health professional (such as the intravenous route), and noninvasive, which can contribute to increasing patient compliance [16,17,18].
These advantages are connected to the unique anatomy of the nasal cavity, which includes a direct connection to the CNS, allowing the drug to be transported to the brain. This makes the nasal cavity the only place in the human body where the nervous system is in direct contact with the surrounding environment. More specifically, the nasal cavity is divided into two parts by the nasal septum, and each part consists of three distinct regions: the vestibule, the olfactory region, and the respiratory region. The vestibule is located at the entrance of the nasal cavity and is responsible for the filtration of inhaled particles. It is the region that least contributes to the absorption of drugs. The respiratory region consists of turbinates, which are responsible for humidification and regulation of the temperature of inhaled air, contributing to the formation of an airflow that improves the contact between inhaled air and the nasal mucosa. The epithelium cells of this region are covered by microvilli and long cilia, which contribute to improved absorption. It is the main responsible region for systemic drug absorption, having a surface area of about 160 cm2 and high vascularization, which provides a high blood flow. From systemic circulation, the drug can be transported to the brain, but in that case, it needs to cross the blood-brain barrier in order to reach the CNS. It can also contribute to the direct absorption of drugs into the CNS through the trigeminal nerve. The olfactory region occupies a surface area of approximately 10 cm2, playing a crucial role in the absorption and transport of drugs to the brain. The olfactory epithelium consists of olfactory nerves, responsible for the direct transport of the drug from the nasal cavity to the brain, using the olfactory nerve. Hence, in short, intranasally administered drugs can reach the site of action in the brain directly, using the olfactory or trigeminal nerves (direct transport), or indirectly, being absorbed into the systemic circulation and then crossing the blood-brain barrier (indirect transport) (Figure 1) [16,17,19,20].
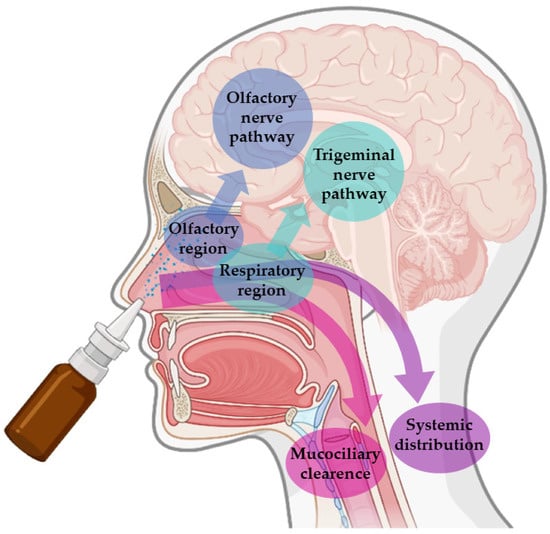
Figure 1. Pathways of brain drug transport after intranasal administration: direct transport (neuronal transport—olfactory nerve and trigeminal nerves) and indirect transport (systemic distribution).
Nevertheless, in spite of the many important advantages that intranasal administration presents, it is necessary to consider some limiting factors that hinder drug absorption, namely: the physical removal of the drug from the nasal cavity by mucociliary clearance mechanisms; enzymatic degradation in the mucus and nasal epithelium layer; and the volume of formulation that can be administered, that is limited to 25–200 μL, which turns this route of administration more appropriate for potent drugs. Mucociliary clearance has the function of protecting the respiratory system against bacteria and inhaled particles as a result of the combined effect of mucus and cilia that transport the particles from the anterior to the posterior region of the nasal cavity, being eliminated to the bottom of the throat. Hence, drug bioavailability is diminished by the high flow of nasal secretions and ciliary movement since these decrease the time of residence of the drugs in the nasal cavity, affecting the permeability through the mucosa. The enzymatic activity in the nasal cavity also constitutes a barrier to the absorption of drugs since several enzymes, such as the various isoforms of cytochrome P450, are present in the nasal mucosa and perform enzymatic degradation of various drugs [17,19,20,21]. The many strategies that have been developed to overcome these obstacles, as well as the factors that influence nasal drug absorption, are addressed in the following Section 1.1.1 and Section 1.1.2.
2. Physicochemical and Formulation Factors That Influence Nasal Drug Absorption
Nasal absorption is influenced by several factors, namely anatomical and physiological factors (mucociliary clearance, enzymatic degradation, membrane transport, mucosal irritation, and deposition), physicochemical factors (molecular drug weight, lipophilicity and ionization state), and formulation factors (type and characteristics of the formulation, volume of administration, drug strength, viscosity, pH, osmolarity). When the drug is administered intranasally, it comes into direct contact with the nasal mucosa. The passage through the mucus layer is the first step to its absorption. Mucin, the main protein of the mucus, binds to drugs, hampering their diffusion. Additionally, alterations in pH or temperature may alter the structure of the mucus, making the diffusion of the drug more difficult. Moreover, the mucus has elastic and viscous properties that influence the transport of drugs; hence, if the mucus is more viscous, mucociliary clearance is reduced, and, therefore, the contact time between the drug and the mucosa is increased, which may contribute to improved drug absorption [17,20,21].
After passing through the mucus layer, the transport of the drug can be performed by different mechanisms that include: transcellular diffusion, in which transport is carried out through the membrane by passive diffusion or active transport; and paracellular diffusion, a passive process, in which the drug moves through the intercellular space. The lipophilicity of the drug is one of the most important factors that determines whether the transport will be performed by transcellular diffusion or paracellular diffusion. Lipophilic drugs are preferably transported through transcellular diffusion, showing fast and efficient absorption when administered intranasally. Hydrophilic drugs are mainly transported via paracellular diffusion, resulting in low absorption. The rate of a drug’s diffusion through the nasal mucosa is also influenced by the state of ionization and molecular weight of the drug. Consequently, lipophilic and uncharged (neutral) drugs with low molecular weight are more easily absorbed when compared to hydrophilic, charged and/or high molecular weight molecules. Absorption also depends on the pKa of the drug and the pH at the absorption site, which presents values between 5.0 and 6.5 in the nasal mucosa. It is also influenced by the solubility of the drug since nose secretions have a more watery nature; therefore, the drug should present an appropriate aqueous solubility for better dissolution in the mucus itself [16,17,21,22].
In order to optimize drug transport to the action site after an intranasal administration, it is necessary to take into account certain characteristics of the formulation, for example, factors such as its capability to adhere to the mucosa, nasal permeability, and drug deposition in the olfactory epithelium. These characteristics should be elevated and should also allow a controlled and constant release of the drug. The excipients of the formulation should be selected, taking into account their functions, with the purpose of assigning properties to the formulation that allow to protect the drug and favor its administration and arrival of the drug at the action site. The excipients must also be compatible with the active substance and non-toxic or irritating to the nasal mucosa [17]. The concentration of the drug in the formulation and the volume of administration should also be taken into account since it is only possible to administer approximately 200 μL of formulation in the nasal cavity, which makes this route of administration suitable for potent drugs but challenging to drugs that require high doses or that have reduced solubility [17,18,23].
The pH of the formulation is also important and should be close to the pH of the nasal cavity to avoid mucosal irritation. Lysozyme found in nose secretions contributes to the dissolution of certain bacteria and helps to maintain acidic pH. Additionally, the pH of the formulation should be selected considering the stability of the drug and should also contribute to the existence of a higher fraction of non-ionized drug, while maintaining the functionality of the excipients. Another very important factor is the viscosity of the formulation, which should ensure contact with the nasal mucosa for an adequate period of time. Higher viscosity increases the time of contact of the drug with the mucosa and may contribute to increased absorption. However, formulations that are excessively viscous can decrease the diffusion of the drug from the formulation itself, reducing its absorption. Gel formulations can be used to increase the time of permanence of the drug in the nasal cavity and, consequently, improve its bioavailability. The most used gelling agents include cellulose derivatives (methylcellulose or carboxymethylcellulose) and carbopol. Also, considering that isotonic solutions are better tolerated, the osmolarity of the formulations should be between 285 and 310 mOsmol/L to avoid mucosal irritation. However, hypertonic solutions can be used since they transiently reduce ciliary activity, which can increase the retention time of the drug in the nasal cavity, promoting its absorption. Nevertheless, caution is required when hypertonic solutions are used in order to ensure that no damage is caused to the nasal mucosa [17,18,24,25,26,27,28,29].
3. Strategies Used to Improve Drug Absorption
Many different strategies have been developed in order to improve drug solubilization and absorption, such as the use of prodrugs, absorption enhancers, enzymatic inhibitors, mucoadhesive agents, and nanometric drug transport systems [30].
Structural changes in the drug molecule can help improve their characteristics by changing physicochemical properties such as molecular weight, partition coefficient, and solubility. These can be used to improve formulation drug strength, absorption through biological barriers, or premature metabolism. The grand majority of prodrugs are administered in their inactive form, requiring biotransformation to become their active form, which produces a pharmacological effect [17,28,29,31,32,33].
Absorption enhancers are excipients that can improve drug permeability, which is especially important for hydrophilic drugs. They act by altering the phospholipid bilayer and membrane fluidity or by alternately opening tight junctions between epithelial cells, improving paracellular transport. The most commonly used are surfactants (e.g., polysorbate, poloxamers), bile salts (e.g., sodium cholate), fatty acids (e.g., stearic acid, palmitic acid), chelators (e.g., ethylenediaminetetraacetic acid (EDTA), salicylates) and polymers (e.g., chitosan, poly(D,L–lacitde co– glycolide) (PLGA)) [17,22,34,35,36].
Enzymatic inhibitors can also be used to protect drugs against the enzymatic degradation that occurs in the nasal cavity, thereby increasing the available fraction of the drug for absorption and, consequently, bioavailability. Peptidase inhibitors and proteases, such as amastatin, boroleucine, bacitracin, and puromycin may be used [22,33,37,38].
Mucoadhesive agents increase the contact time between the formulation and the nasal mucosa, also favoring the paracellular transport of hydrophilic drugs and reducing mucociliary clearance by establishing a connection between mucin and a polymer. One of the possible mechanisms adopted by the mucoadhesive systems is the absorption of water from the nasal mucosa, which leads to the swelling of the polymer, and consequent penetration into the mucus with fixation of the formulation to the nasal cavity, improving the absorption of the drug. An example is chitosan, a biocompatible and biodegradable polymer, is widely used not only for its mucoadhesive properties but also for increasing permeability and paracellular transport, through interaction with the tight junctions [17,30,39,40,41].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14122742
This entry is offline, you can click here to edit this entry!
