Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Rheumatology
Understanding the basis of osteoarthritis (OA) has seen some interesting advancements. It has been observed that cartilage degeneration is preceded by subchondral bone lesions, suggesting a key role of this mechanism within the pathogenesis and progression of OA, as well as the formation of ectopic bone and osteophytes. Moreover, low-grade, chronic inflammation of the synovial lining has gained a central role in the definition of OA physiopathology, and central immunological mechanisms, innate but also adaptive, are considered crucial in driving inflammation and tissue destruction.
- osteoarthritis
- physiopathology
- subchondral bone
1. Introduction
Osteoarthritis (OA) is the most common chronic articular disease with an increasing prevalence due to population aging and obesity [1,2,3]. Osteoarthritis is characterized by articular cartilage degeneration and persistent pain, causing disability, loss of function, decreased quality of life (QoL), and economic burden [4,5,6].
The global incidence of OA has been estimated at about 20% [7]. However, according to recent assessments, regional and individual country differences exist [7,8,9]. Indeed, OA incidence has been estimated at 10–17% in Europe, 12–21% in North America, 2–4% in South America, 16–29% in Asia, Africa, and Middle-Eastern countries [9,10]. Considering that OA susceptibility is strongly affected by genetic and environmental risk factors, the assessment of OA epidemiology in different populations can contribute to the understanding of the worldwide burden of the disease and may also shed light on the underlying mechanisms of the disease.
Osteoarthritis has long been considered a degenerative cartilage disease, characterized by a progressive loss of functionality due to different factors, such as excessive body weight, advanced age, surgical joint treatments, repeated joint injuries, and genetic predisposition [11,12]. Nevertheless, the understanding of the basis of OA has seen some interesting advancements in recent years [1,13,14]. Indeed, modern imaging approaches have shown that OA pathogenesis involves the breakdown of cartilage and structural changes in the whole joint [1,6,14]. In particular, it has been observed that cartilage degeneration is preceded by subchondral bone lesions, suggesting a key role of this mechanism within the pathogenesis and progression of OA, as well as the formation of ectopic bone and osteophytes [5,12,15,16,17].
Moreover, low-grade, chronic inflammation of the synovial lining now plays a central role in defining OA pathophysiology, and innate but adaptive central immunological mechanisms are now considered crucial in driving inflammation and tissue destruction. [3,14]. Lastly, the role of neuroinflammation and central sensitization mechanisms as underlying causes of pain chronicity has been characterized [18,19].
This evidence has led to a renewed definition of OA, which is now intended as a complex multifactorial joint pathology caused by inflammatory and metabolic factors underlying joint damage [1,3,6,14]. This new perspective directly impacts the definition of the correct therapeutic approach to OA. Therefore, an improved understanding of these pathophysiological mechanisms is crucial.
2. Changes in the Osteochondral Unit during Osteoarthritis: The Role of the Subchondral Bone
Increasing evidence suggests that OA is a whole-joint disease in which all the joint components (cartilage, synovium, subchondral bone, and associated muscles) are affected [1,3,6] Articular cartilage covers the ends of bones in the diarthrodial joint and absorbs shock from joint movement. It has an aneural, avascular, and lymphatic structure composed of 65–80% water, 20–40% extracellular matrix (ECM), and 1–5% chondrocytes [20,21].
The subchondral bone is the bony layer below the hyaline cartilage and can be divided into the subchondral bone plate (SBP) and subchondral bone trabeculae (SBT). The SBP is a compact, porous, calcified plate traversed by nerve fibers and vessels. The SBTs are cancellous bony structures that undergo continuous bone remodeling [5,21]. Articular cartilage, calcified cartilage, SBP, and SBTs form the osteochondral unit, which transfers load during joint movement (Figure 1) [20,22]. Subchondral bone provides both nutritional and mechanical support for cartilage within the osteochondral unit, indicating that changes in the subchondral bone microenvironment can affect cartilage metabolism [5,17,20,22].
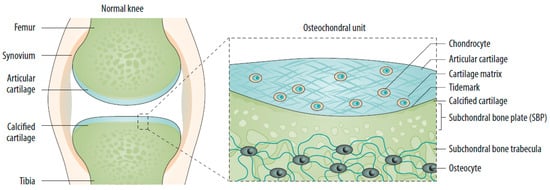
Figure 1. The normal joint—e.g., the knee—is composed of two articulating bones (femur and tibia), the articular cartilage, and the synovial lining of the joint cavity. A thin layer of calcified cartilage is present underneath the articular cartilage. The subchondral bone beneath the calcified cartilage is formed from cortical bone that merges into a network of trabecular bone, which is relatively porous and metabolically active. Source: original.
Subchondral bone marrow lesions (SBMLs), reported by abnormal MRI signals below the calcified cartilage, have affected more than half of asymptomatic individuals over 50 years. Their prevalence seems to increase with age [5]. The SBMLs are due to abnormal and persistent mechanical insults, which lead to cellular and biomolecular responses to microfractures. Since SBMLs can be observed in the early stage of OA, and the worsening of SBMLs based on MRI manifestations has been associated with subsequent radiographic findings and persistent pain, they are thought to be helpful in early screening [5,17,23]. The site of an SBML is characterized by a high in situ turnover rate, pain, and activation of proinflammatory pathways, finally resulting in increased subchondral sclerosis and bone mineral density [5,18]. Clinical observations over 24 months suggested a strong relationship between the increased size of the SBMLs and cartilage volume loss in corresponding regions [5,17]. Consequently, subchondral bone remodeling is now considered a key element of OA, which can disrupt the integrity of the osteochondral unit and lead to increased crosstalk between cartilage and subchondral bone (Figure 2) [5,17,22]. A recent study reported the presence of a horizontal fissure at the interface within the osteochondral unit in obese patients, characterized by irregular cartilage erosion, fibro-granulation tissue infiltration, presence of free cartilage/bone debris, and rupture of micro capillaries [24]. This can be considered a new type of pathological feature, where neurovascular invasions have also been identified in degenerative osteochondral tissues (see following sections).
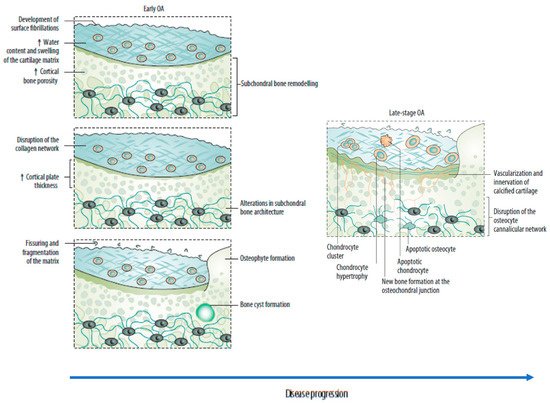
Figure 2. Sequential changes in the osteochondral unit during the evolution of osteoarthritis. (Left) Early OA is characterized by increased remodeling of the subchondral bone plate. With disease progression, loss of cartilage matrix proteoglycans and erosion of the collagen network led to the development of deep fissures and delamination of the cartilage, with exposure of the underlying zones of calcified cartilage and subchondral bone. In the subchondral bone, cortical plate thickness gradually increases. (Right) Chondrocytes exist mostly in clusters in late-stage OA, but chondrocyte apoptosis is also evident. In the deeper zones, chondrocytes undergo phenotypic alterations, developing features of a hypertrophic phenotype. The calcified cartilage expands and advances into the overlying hyaline articular cartilage, with duplication of the tidemark. This process is initiated by penetrating vascular elements, and accompanying sensory and sympathetic nerves, into the osteochondral junction. OA: osteoarthritis. Source: original.
3. Risk Factors
The risk factors for OA can be divided into individual susceptibility features (increasing age, obesity, female sex, joint biomechanics, genetic factors) and factors that alter the biomechanical stability of joints (injury, repetitive joint use through occupation or leisure, and joint malalignment) [11,12,25].
3.1. Individual Risk Factors
The increasing incidence of OA with age can be related to cumulative exposure to various risk factors and age-related biological changes in the joint structures [2,26]. Female sex and obesity have been strongly correlated with knee OA [27]. Knee malalignment and knee extensor muscle weakness have also been defined as moderate to strong risk factors [28].
Female sex and obesity are less represented risk factors for hip OA, but cam deformity or acetabular dysplasia have been found to moderately-to-strongly increase OA risk [6] Cam deformity and mild dysplasia have been found to increase the risk of OA, especially in middle-aged (55–65 years) patients; otherwise, a strong association between hip OA and severe dysplasia has been described, leading to its development at an early age (<50 years) [29].
Some studies supported the theory of diabetes as an independent risk factor for OA. One study, examining metabolic risk factors and their role in knee OA, showed a significant association between impaired glucose tolerance and knee OA after adjusting for age and biological sex, but not BMI [30,31,32]. In contrast, other more recent studies showed no association between diabetes and OA [33,34,35]. The differences in the results obtained across these studies can be attributed to other OA common risk factors, such as obesity and aging. However, these findings have not conclusively established the exact role diabetes plays in OA.
3.2. Genetics
The contribution of genetics in OA is estimated to be between 40% and 80%, with a stronger genetic contribution in hand and hip OA than knee OA [25,36]. Rare mutations in monogenetic disorders associated with OA can result in early-onset OA. In contrast, late-onset OA is often characterized by a multifactorial clinical picture composed of common DNA variants and other risk factors. The effect size of these common variants is generally small [21,26]. Of note, among the 70 putative genes identified in genome-wide association studies in OA, no inflammatory genes can be detected; otherwise, growth factor clusters are strongly represented [37,38]. These include variants in TGF-β family genes, including ligands (TGFB1, GDF5), latent binding proteins (LTBP1, LTBP3), and signaling molecules (SMAD3). The FGF family is also represented. Collectively, these results underline the role of the loss of reparative features within the joint in the development of OA [39].
4. Joint-Related Factors
Heavy work activities are risk factors for both hip and knee OA; employment in farming or the construction industry is especially associated with hip OA, and work that involves frequent kneeling and heavy lifting is associated with knee OA [27].
Increased risk of OA has been found among athletes active in different sports [40]. Several high-impact sports (e.g., football, handball, hockey, wrestling, weight-lifting, and long-distance running) have been reported as moderately to strongly associated with an increased risk of hip or knee OA, often with a dose-response dependency [41,42]. For knee OA, the increased risk with sport is partly because of knee injuries; for hip OA, the risk might be associated with cam impingement, which can develop during sporting activities in adolescents [6]. Otherwise, in the first study examining the association between objectively measured physical activity and risk of developing knee OA in a community-based cohort of middle-aged and older adults at high risk for symptomatic knee OA, moderate–vigorous physical activity was not associated with incident knee OA [43].
Lastly, an association between the increasing use of technology, computers, and smartphones and hand OA has yet to be proved but is a common concern that requires further investigation, considering the increasing prevalence of these technologies in our lives [4].
This entry is adapted from the peer-reviewed paper 10.3390/jcm11206013
This entry is offline, you can click here to edit this entry!
