1. Introduction
Inflammatory bowel disease (IBD), generally including ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic and relapsing intestinal inflammatory disease. There are differences in the predilection sites between UC and CD. UC usually involves the distal colon and rectum, invading the intestinal mucosa and submucosa, while CD, a discontinuous inflammation disease, can attack the entire digestive tract, and its lesions can penetrate all the intestinal layers [1]. IBD tends to occur in developed countries, and epidemiological surveys show that its incidence has gradually increased in recent years globally. It was reported that, from 1980 to 2013, the incidence of IBD in Denmark increased from 15.9 to 27.7 per 100,000 people [2]. Furthermore, the incidence of IBD in the Netherlands increased from 17.51 to 38.96 per 100,000 people from 1991 to 2010, with an average growth rate of 4.30% annually [3]. In recent years, the prevalence of IBD has increased in Asia, and it shows a gradient trend of increase from north to south in China [4]. Although the pathogenesis of IBD is still elusive, it is generally believed to be related to genetic susceptibility, environmental factors, immune dysfunction, and gut microbiota [5,6]. It was reported that a high risk of developing IBD was observed in the offspring of affected parents [7]. Potentially relevant environmental factors, such as antibiotic exposure, smoking, dietary fiber, saturated fats, and major life stressors, are associated with IBD incidence [8]. In addition, gut microbiota dysbiosis was demonstrated in IBD patients and consistently characterized by a reduction in microbiota diversity compared with healthy individuals [9]. The use of existing therapeutic drugs, including sulfasalazine, mesalazine, glucocorticoids, and biological drugs, is limited to some extent due to their modest effects, adverse reactions, and high prices [10]. Therefore, new treatment strategies and methods have been called for in recent years.
Gut microbiota is widely involved in host metabolic activities, producing a variety of active metabolites that play important roles in maintaining a host’s intestinal barrier integrity and immune balance via providing nutrition to intestinal epithelial cells and activating various receptors directly or indirectly. For example, gut microbiota is widely involved in the metabolism of carbohydrates, as well as tryptophan and bile acids (BAs), to generate short-chain fatty acids (SCFAs), indole derivatives, and secondary bile acid. Furthermore, gut microbiota helps to promote the biosynthesis of vitamin B and vitamin K in a host, showing a protective effect for the host [
62].
In recent years, more and more studies have shown that the disturbance of the gut microbiota metabolic process is related to the occurrence and development of IBD. It is worth noticing that the contents of SCFAs in the intestines of IBD patients were significantly reduced compared with those of healthy people [
63]. Studies have also found that the content of microbial-derived aryl hydrocarbon receptor (AhR) agonists is significantly reduced in IBD patients. In addition, supplementation with AhR agonists could significantly improve intestinal barrier integrity and ameliorate IBD symptoms [
64]. There are increasing studies focusing on the role of gut microbiota in diseases, and alteration in the gut microbiota metabolic process is tightly associated with IBD (
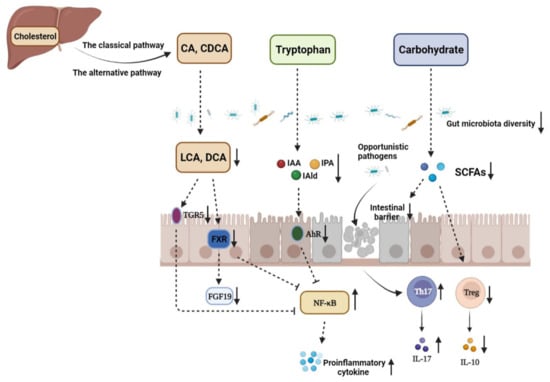
Figure 1).
Figure 1. The disturbance of gut microbiota and microbiome metabolites in the pathogenesis of IBD. CA: cholic acid; CDCA: chenodeoxycholic acid; LCA: lithocholic acid; DCA: deoxycholic acid; TGR5: G-protein-coupled bile acid receptor 1; FXR: farnesoid X receptor; FGF19: fibroblast growth factor 19; IAA: indole-3-acetic acid; IAld: indole-3-aldehyde; IPA: indole-3-propionic acid; AhR: aryl hydrocarbon receptor; SCFAs: short-chain fatty acids. The decrease in the abundance and diversity of gut microbiota weakens the intestinal microbial barrier in IBD, which provides opportunistic pathogens an opportunity to invade gut mucosa and induce imbalance between Th17 and Treg cells, thus aggravating intestinal inflammation. The contents of metabolites, such as SCFAs, indole derivatives, and secondary BAs, derived from carbohydrates, tryptophan, and primary BAs under the action of the gut microbiota are significantly decreased due to gut microbiota dysbiosis. SCFAs mediate diverse effects on mucosal immunity, such as the maintenance of mucosal integrity, by supplying an energy source to colonocytes and expending Treg cell proportions. Therefore, SCFA reduction can aggravate intestinal injury in IBD patients. In addition, dysbiosis leads to loss of the microbial activation of tryptophan, which makes the endogenous ligands AhR, IAA, IPA, and IAld decreased. Gut microbiota disturbance in IBD can also decrease the contents of secondary BAs in the colon, such as LCA and DCA, which downregulates the activation of TGR5 and FXR and enhances NF-κB transcription, respectively.
2. SCFAs and IBD
The gut microbiota mainly depends on undigested food in the upper gastrointestinal tract to survive. Dietary carbohydrates are mainly fermented into SCFAs and gases by the gut microbiota. The three most common SCFAs in the gut are acetate, propionate, and butyrate. Among the SCFAs, butyrate is considered to be the most important component for human health as not only the main energy source of colonocytes, but also an important regulator of intestinal barrier integrity. In addition, butyrate promoted intestinal epithelial cell differentiation, tissue development, and immune balance [
65]. Acetate is produced by many bacteria during food fermentation, while propionate and butyrate tend to be produced by specific bacteria. Firmicutes are the primary butyrate-producing bacteria, which include
Lachnospiraceae and
F. prausnitzii [
62]. Evidence from 16S bacteria sequencing demonstrated that the abundances of
Lachnospiraceae and
F. prausnitzii were significantly reduced in the feces of IBD patients, resulting in decreased intestinal butyrate content and aggravated intestinal barrier damage in inflamed colons [
66,
67]. In addition, a relevant study showed that the abundance of
Roseburia hominis, a butyrate-producing bacteria, was notably reduced in IBD patients, which was tightly related to the reduced content of SCFAs in the feces of patients [
68]. A meta-analysis including eleven studies demonstrated that UC patients had dramatically lower total SCFA concentrations compared to healthy subjects, and the concentrations of acetate, propionate, and butyrate were different according to disease status. Active UC patients had reduced acetate and propionate concentrations, while UC patients in remission had similar concentrations to healthy subjects [
69].
SCFAs are capable of promoting the differentiation and development of Treg cells by inducing Foxp3 expression or inducing DCs and intestinal epithelial cells to produce retinoic acids and TGF-β1, thus having a potential advantage in exerting anti-inflammatory effects in gut inflammation [
70]. Similarly, in a randomized double-blind clinical trial, a topical application of SCFAs was proved to reduce endoscopic and histopathological scores and relieve inflammatory symptoms in UC patients [
71]. TJPs play an important role in the maintenance of intestinal barrier integrity by strengthening the connection between intestinal epithelial cells and promoting the polarization of enterocytes. Many studies have shown that SCFAs, especially butyrate, can enhance the expressions of TJPs, such as Claudin-1 and ZO-1 in the colon, which are significantly decreased in the intestines of IBD patients [
72]. As regulators of gut microbiota dysbiosis, probiotics and prebiotics are supposed to improve the diversity of gut microbiota and increase the relative abundance of SCFA-producing bacteria, resulting in alleviated severity of IBD [
73,
74].
3. Tryptophan Metabolism and IBD
Tryptophan is an essential aromatic amino acid for the human body, and a host mainly obtains tryptophan from external intake. Tryptophan plays an important role in a host as a synthetic precursor of various biologically active substances, such as serotonin (5-HT), kynurenine (Kyn), and indole derivatives [
75]. There are three main metabolic pathways of tryptophan in the body [
76]. The first one is the Kyn pathway (KP), through which tryptophan is metabolized into kynurenine (Kyn) by indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO). In the second pathway, tryptophan is metabolized into 5-HT by the tryptophan hydroxylase 1 enzyme (TPH1) in enterochromaffin cells. The third is the microbial pathway, where tryptophan is converted into indole and its derivatives under the action of the gut microbiota (
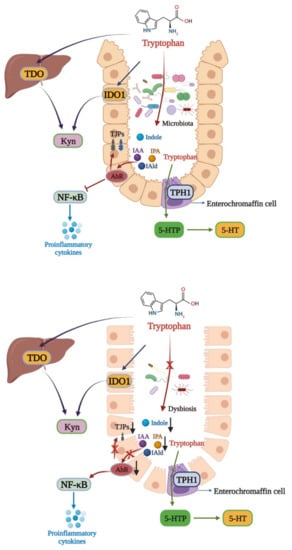
Figure 2). Indoles act as endogenous ligands for AhR and can activate AhR to exert a wide range of physiological effects [
75]. Due to their wide distribution and complex mechanisms, AhR and its endogenous ligands have become a research hotspot in recent years.
Figure 2. Tryptophan metabolism disturbance in IBD. There are three main metabolic pathways of tryptophan. The major pathway is the Kyn pathway, with tryptophan also being metabolized into indole derivatives. In healthy individuals, the gut microbiota metabolize tryptophan into IAA, IPA, and IAld, which can activate AhR to exert a protective effect in the colon via inhibiting NF-κB and increasing TJPs expressions. In IBD, gut microbiota dysbiosis leads to the microbial activation of tryptophan, aggravating intestinal inflammation. TPH1: tryptophan hydroxylase 1; 5-HTtp: 5-hydroxytryptophan.
AhR is a member of the PER-ARNT-SIM (PAS) superfamily of transcription factors, which sense changes in the cellular environment and regulate the physiological balance of the body. It was reported that AhR inhibited the expression of NF-κB in a manner dependent on suppressor of cytokine signaling 2 (SOCS2) after activation to exert anti-inflammatory activity [
77]. In addition, AhR maintains the integrity of intestinal and skin barrier activation by increasing the expressions of intestinal TJPs, such as ZO-1 and occludin, or activating the AhR-Nrf2 pathway [
78,
79]. Tryptophan can be decomposed into indoles and their derivatives under the action of the gut microbiota, such as IAA, IPA, and IAld [
62]. These indole derivatives are able to activate AhR to protect the intestinal barrier and reduce the expressions of intestinal proinflammatory cytokines.
In recent years, more and more studies have shown that disorders of tryptophan metabolism are strongly related to IBD [
80,
81]. In a clinical study, it was found that plasma tryptophan levels were decreased in IBD patients, and plasma tryptophan concentrations were negatively correlated with IBD severity [
82]. Furthermore, the contents of AhR ligands derived from gut microbiota metabolism were significantly reduced in IBD patients, indicating the importance of endogenous AhR ligands in intestinal inflammation [
83]. A similar finding was also revealed in AhR-knockout mice. Nikolaus et al. demonstrated that AhR-knockout mice were more sensitive to DSS-induced colitis, and supplementation with AhR ligands could improve the symptoms of colitis in mice [
84]. Microbial-derived AhR ligands, such as IAA, IPA, and IAld, could activate AhR to reduce intestinal inflammation via inhibiting NF-κB signaling pathway activation and TNF-α expression in the intestines of IBD mice [
85]. In addition, AhR activation was reported to increase the expressions of TJPs and promote wound repair in the intestinal tracts of UC mice [
86].
As mentioned above, the gut microbiota is the primary source of endogenous AhR ligands. Some AhR ligands producing commensal microbiomes, such as
Peptostreptococcus russellii,
Lactobacillus, and
Bifidobacteria, have been proved to be decreased in IBD patients [
75,
87]. Furthermore, Natividad et al. found that the administration of a
Lactobacillus strain with high tryptophan-metabolizing capabilities could improve impaired microbiota-derived AhR ligand signaling in a host [
88]. In general, gut microbiota dysbiosis induces tryptophan metabolite alteration to aggravate IBD progression, which is mainly based on reduced indole derivatives and AhR activity. However, due to the complexity of the gut microbiota, the microbiomes participating in tryptophan metabolism still deserve identification in the future.
4. Bile Acids and IBD
BAs are synthesized from cholesterol in hepatocytes, and there are two main synthetic pathways, namely the classical pathway mediated by CYP7A1 and the alternative pathway mediated by CYP27A1, that generate primary BAs, such as CA and CDCA. Primary BAs are stored in the gallbladder and are subsequently secreted in the gut after conjugation to glycine or taurine. They can be transformed into secondary BAs under the action of the gut microbiota [
89]. Secondary BAs, such as LCA and DCA, act as high-affinity ligands for TGR5 and FXR, the activation of which exerts immunomodulatory and anti-inflammatory effects [
90]. Generally, there is a balance between primary BAs and secondary Bas, but the balance can be broken by gut microbiota dysbiosis, which causes increased primary BAs and decreased secondary BAs, inducing a state of inflammation in the colon (
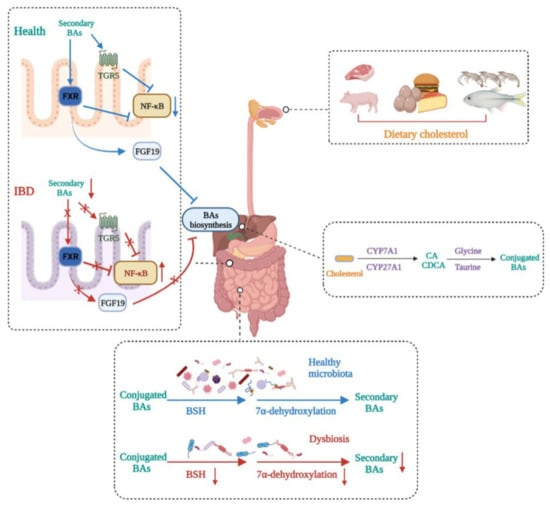
Figure 3).
Figure 3. Bile acid metabolism disturbance in IBD. BA metabolism is altered in IBD patients. Dietary cholesterol is digested and absorbed in the gastrointestinal tract. Cholesterol is biotransformed to conjugated primary BAs in the liver and then secreted in the gut. Gut microbiota dysbiosis in IBD impairs BSH activity and 7α-dehydroxylation, leading to decreased secondary BAs. This induces decreases in the expressions of FXR and TGR5, which makes the transcription of NF-κB relevantly enhanced, thus aggravating IBD severity. In addition, decreased secondary BAs caused by dysbiosis reduce the production of FGF19, leading to primary BA accumulation in the liver.
A growing number of studies have shown that disturbances in bile acid metabolism are associated with IBD. Evidence from BA profiles found enhanced conjugated primary fecal BAs and decreased secondary fecal BAs in IBD patients, which could be caused by impaired deconjugation, transformation, or desulphation activities of the microbiota in IBD patients [
91]. As high-affinity ligands for TGR5 and FXR, this BA alteration could lower the activation of TGR5 and FXR, which is detrimental to exerting an anti-inflammatory role. Meanwhile, the activation of FXR upregulated the expression of FGF19 in humans, a substance supposed to reach the liver and inhibit the synthesis of BAs, thus decreasing its toxicity effect on tissues [
92]. Furthermore, it was reported that FXR and TGR5 activation showed anti-inflammatory effects by binding directly to an NF-κB p65 subunit to inhibit its transcription [
89]. Macrophages, including M1 and M2 macrophages, are the main regulators of cytokine production in the gastrointestinal tract. TGR5 is a cell membrane receptor containing seven transmembrane domains, and the activation of TGR5 induces different reactions in M1 and M2 macrophages. The activation of TGR5 in M2 macrophages promoted immunosuppression by producing IL-10, leading to decreases in TNF-α and IFNγ [
93]. However, TGR5 activation in M1 macrophages exerted the opposite effect and induced inflammation by strengthening NF-κB transcription and promoting proinflammatory cytokine production [
89]. Despite the different roles of TGR5 in M1 and M2 macrophages, TGR5 activation exerts an anti-inflammation effect overall in macrophages.
The gut microbiota is widely involved in transforming BAs into unconjugated secondary Bas. Generally, conjugated Bas are converted into respective unconjugated free forms through the action of bile salt hydrolase (BSH)-carrying bacteria, such as
Bacteroides,
Clostridium, Lactobacillus,
Bifidobacterium, and
Listeria, the abundances of which have been demonstrated to decrease in IBD patients [
50,
89]. Subsequently, secondary BAs are formed from unconjugated BAs through the 7α-dehydroxylation of commensal bacteria, such as
Bacteroides,
Clostridium,
Eubacterium, and
Lactobacillus [
81]. Therefore, gut microbiota dysbiosis occurring in IBD patients can cause BA metabolism alteration, mainly manifested as increased primary BAs and decreased secondary BAs, which aggravates IBD progression by inhibiting FXR and TGR5 activity. Therefore, the manipulation of BA balance shows great potential for IBD treatment.
This entry is adapted from the peer-reviewed paper 10.3390/nu14235140