Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Circular RNAs (circRNAs) are an abundant class of endogenous non-coding RNAs (ncRNAs) generated from exonic, intronic, or untranslated regions of protein-coding genes or intergenic regions. The diverse, stable, and specific expression patterns of circRNAs and their possible functions through cis/trans regulation and protein-coding mechanisms make circRNA a research hotspot in various biological and pathological processes. It also shows practical value as biomarkers, diagnostic indicators, and therapeutic targets.
- circRNAs
- ovary
- follicle development
1. Background
The genome-wide profiles of ovarian circRNAs were mainly reported in humans, mice, pigs, and goats. In humans, circRNA studies have been focused on pathological examination of ovarian cancer, polycystic ovarian syndrome (PCOS), and ageing. Luckily, studies in animals provided more knowledge regarding ovary growth, changes in estrus, as well as follicle development, and atresia. Comparisons between different reproductive performances and breeds were also reported (Figure 1). Here, the researchers reviewed the global studies of each field first and summarized the proven function of individual circRNAs in Table 1.
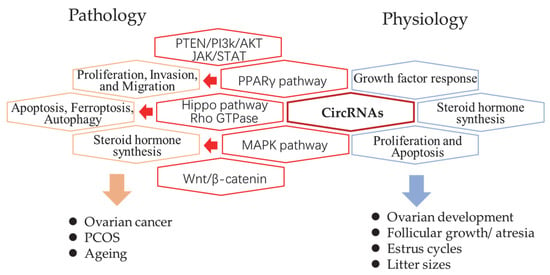
Figure 1. The summary of circRNAs related pathways in pathology and physiology processes of the mammalian ovary. The revealed circRNA-related signaling pathways (red hexagon) and their related biological processes in pathology (orange hexagon) and physiology (blue hexagon).
2. CircRNAs in Ovarian Cancer
CircRNAs in ovarian dysfunction attracted close attention due to their tight interaction with miRNAs. In 2015, a comprehensive assessment compared circRNA levels across several normal and cancerous tissues, including ovarian cancer, and discovered a global reduction in circular RNA abundance in cancer compared to normal tissues, therefore suggesting a negative correlation between circular RNA abundance and cell proliferation [1]. Ning et al. also performed circRNA-sequencing in epithelial ovarian cancer (EOC) and normal ovarian tissues and identified 4388 differently expressed circRNAs [2]. Almost simultaneously, Teng et al. analyzed circRNA expression profiles in EOC and normal ovarian tissues, in which the expressions of 5551 circRNAs were differentially expressed [3]. Gao et al. sequenced and compared circRNA in high-grade serous ovarian cancer (HGSOC) specimens and normal ovarian tissues. Among 710 differentially expressed circRNAs, circRNA1656 was confirmed down-regulated in HGSOC tissues and ovarian cancer cell lines [4]. Furthermore, Zhao et al. investigated the expression of circRNAs in paired cisplatin-sensitive and cisplatin-resistant tissues of ovarian cancer by microarray analysis and reported 339 aberrantly expressed circRNAs [5]. Cdr1as was proven to sensitize ovarian cancer to cisplatin by regulating the miR-1270/SCAI axis. Based on these high-throughput studies, a detailed functional analysis in single-circRNA level was reported in continuance, which showed great potential as biomarkers for ovarian cancer.
3. CircRNAs in PCOS
PCOS is the most common endocrine disorder in women of reproductive age. To reveal the functions of circRNAs in the development of PCOS, circRNA profiles from cumulus cells and follicle fluid were assessed, respectively. Che et al. determined 311 increased and 721 decreased circRNAs in cumulus cells from PCOS compared to control participants who underwent IVF using microarray [6]. With these data, Li et al. further combined data of microRNA and mRNA in PCOS to predict circRNAs which may serve as RBP regulators or miRNA sponges [7]. This research conducted a weighted correlation network analysis (WGCNA) to mine PCOS-associated circRNA-miRNA-gene networks and circRNA-RNA binding protein (RBP) networks. Moreover, Wang et al. performed a delicate study by sequencing ribosomal RNA-depleted total RNA from exosomes of follicle fluids. They identified 167 up-regulated and 245 down-regulated circRNAs in PCOS patients [8].
4. CircRNAs during Maternal Ageing
The decline of female reproductive capacity with age, termed ovarian senescence, results in a gradual reduction in the quantity and quality of oocytes. Cheng et al. first compared circRNAs in GCs from in vitro fertilization (IVF) patients with young (≤30) and advanced (≥38) ages using human circRNA microarrays. This research revealed 46 up-regulated and 11 down-regulated circRNAs in aged samples. Later, Cai et al. compared circRNA expression profiles between healthy ovarian cortex from young (25–28) and ageing (44–46) groups and identified 194 up-regulated and 207 down-regulated circRNAs enriched in oxidation-reduction, steroid hormone biosynthesis, and insulin secretion pathways, during ageing [9].
5. CircRNAs and Ovary Development
CircRNA profiles during ovarian development, estrus cycles, and follicular growth were explored mainly using large animals such as pigs and goats. CircRNA landscape in adult and neonatal ovaries was first examined and compared in mouse ovarian tissue using high-throughput sequencing. Estrogen signaling was found to be the most significant pathway that up-regulates in adult ovaries [10]. In pigs, more specifically, ovarian circRNA profiles at three developmental stages (0, 30, and 240 days after birth) were identified and compared with other eight tissues (heart, liver, spleen, lung, kidney, testis, skeletal muscle, and fat). This research revealed ovary-specific/enhanced circRNAs and provided valuable resources for ovarian circRNA study [11]. In addition, the profiles of ovarian circRNAs across pre-, in-, and post-pubertal stages were reported. The research identified 631 stage-specific circRNAs generated from genes involved in steroid biosynthesis, progesterone-mediated oocyte maturation, and autophagy [12].
Regarding the estrus cycle, Liu et al. analyzed the circRNA profiles of Yunshang black goat ovarian tissues among high and low fecundity groups in the follicular phase and luteal phase, and conclude that circRNAs play a key role in both the prolificacy trait and transformation of the follicular phase to the luteal phase in the estrus cycle [13]. At the follicle level, Xu et al. reported 290 differentially expressed circRNAs between large (diameter > 4 mm) and small (diameter < 4 mm) follicles in Dazu black goats. This research also simultaneously generated profiles of mRNAs, long non-coding RNAs (lncRNAs), and microRNAs (miRNAs), creating a good start and helpful reference for integrated ncRNA study during follicle development [14]. To explore the roles of circRNA in growth factors response, Fu et al. [15] profiled circRNAs of bovine cumulus cells treated with or without growth factors (bone morphogenetic protein 15 (BMP15), growth differentiation factor 9 (GDF9), and BMP15+GDF9). This research suggested that GDF9 induced a more significant circRNA shift than BMP15, and BMP15 may play a role in assisting GDF9. Changed circRNAs were involved in pathways, including thyroid hormone signaling ubiquinone and terpenoid-quinones, which affected the proliferation and apoptosis of CCs.
6. CircRNAs and Follicular Atresia
circRNA profiles in healthy and atretic antral follicles were first deep sequenced by Guo et al., and 192 circRNAs were reported to be differentially expressed during the atresia process [16]. Based on this research, detailed functions of circRNAs serving as miRNA sponges in the connective tissue growth factor (CTGF) regulatory pathway [16], inhibin–activin balance [17], and cell viability [18] have been reported. It is widely accepted that GCs play a significant role in the follicular development and atresia processes, thus determining the fate of follicles [19]. Therefore, Meng et al. performed a more specific study to profile circRNAs generated from porcine granulosa cells isolated from healthy atretic antral follicles [20], which is a perfect supplement and advancement to the earlier research. This research further confirmed circRNA functions in oxidative stress inhibition and cell apoptosis pathways.
7. CircRNA and High Reproductive Traits
To explore the circRNA functions in reproductive performance, the circRNA function in litter size was investigated in pigs, goats, and sheep. In pigs, circRNA profiles of ovaries from large and small litter sizes groups were performed by Xu et al. [21], and 56 down-regulated and 54 up-regulated circRNAs were observed in the large litter sizes group. Parallelly, a similar study of ovaries from MeiShan (local breed with large litter) and Large White pigs was performed and revealed 37 up-regulated and 48 down-regulated circRNAs [22]. The pre-ovulatory follicles of the Boer goat and Macheng black goat, which is highly fertile with a twin and multiparous lamb rate of 70%, were compared [23]. This research not only examined goat ovarian circRNA profile for the first time but also identified 37 differentially expressed circRNAs in high litter size breeds. A more delicate analysis was performed in ovarian tissues from both follicular and luteal phases of Harper sheep that were either consecutive monotocous or polytomous. Totals of 183 and 34 differentially expressed circRNAs were identified in h follicular and luteal phases, respectively, and TGF-β and thyroid hormone signaling were highlighted to affect the litter size through circRNAs [24]. However, all these studies suggested that in the ovary, the number of circRNAs that varies between breeds or reproductive performance is relatively low. During the follicle cycle, destined ovarian follicles grow rapidly, which is based on the rapid division of granulosa cells. Therefore, such observations agree with the speculation of a negative correlation between circRNA levels and cell division rate in cancer studies. Moreover, an interesting study in rats revealed potential functions of circRNAs in continuous light-induced ovarian dysfunction, which provided novel clues of circRNA shift in response to temporary environmental changes [25].
Table 1. Circular RNAs and their role in the different ovaries.
| Species | Tissue | CircRNA | Target miRNA/Gene/Protein |
Function | Ref. |
|---|---|---|---|---|---|
| human | OC | Cdr1as | miR-1270/SCAI | sensitizes ovarian cancer to cisplatin | [5] |
| circ-ITCH | miR-145/RASA1 | inhibit tumour progression | [26] | ||
| has_circ_0051240 | miR-637/KLK4 | suppresses cell proliferation, migration, and invasion | [27] | ||
| circEPSTI1 | miR-942 | inhibit cell growth and invasion, induces apoptosis | [28] | ||
| circRNA CDR1 | miR-135b-5p/HIF1AN | decreasing the occurrence and progression of ovarian cancer | [29] | ||
| circLARP4 | down-regulated in cancerous ovarian cells | [30] | |||
| hsa_circ_0007444 | miR-23a-3p/DICER1 | [31] | |||
| circPLEKHM3 | miR-320a/SMG1 | exacerbated the effect of curcumin on ovarian cancer cell proliferation and apoptosis, as well as the anti-tumour effect | [32] | ||
| circABCB10 | miR-1271 | promotes cell proliferation and invasion but inhibits apoptosis | [33] | ||
| circRNA1656 | miR-1301-3p/miR-4660-SIRT3 | down-regulated in HGSOC | [4] | ||
| circ-CSPP1 | miR-1236-3p | promotes proliferation, invasion, and migration | [34] | ||
| has-circ-001567 | promotes cell proliferation and invasion | [35] | |||
| circ-SMAD7 | KLF6 | promotes cell proliferation and invasion | [36] | ||
| circ_0025033 | miR-184/LSM4 | promotes the progression of ovarian cancer | [37] | ||
| circHIPK3 | related to cell growth, migration, and apoptosis | [3] | |||
| PCOS | circ_0023942 | CDK-4 | inhibit granulosa cell proliferation | [38] | |
| circ_0043533 | miR-1179 | related to Bcl-2, CDK2, and Cyclin D1 | [39] | ||
| circ_RANBP9 | miRNA-136-5p/XIAP | exacerbates POS | [40] | ||
| circASPH | miR-375/MAP2K6 | promotes cells proliferation | [41] | ||
| circRHBG | miR-515/SLC7A11 | knockdown of circRHBG promotes ferroptosis in PCOS | [42] | ||
| circ_0005925 | miR-324-3p/MAP2K6 | Promotes Granulosa Cell Growth | [43] | ||
| circ_0043532 | miR-182/SGK3 | promote cell proliferation | [44] | ||
| ovary | circDDX10 | ovarian aging | [9] | ||
| KGN | circUSP36 | PTBP1/NEDD4L | enhance autophagic granulosa cell death | [45] | |
| GCs | circDDX10 | affecting the proliferation and apoptosis and steroid hormone synthesis | [46] | ||
| Pig | ovary | circ-TCP11 | miR-183 | associated with swine litter size | [21] |
| ovary | circSCIN | miR-133, miR-148a/b | affecting estrogen secretion | [22] | |
| GCs | ssc-circINHA-001 | miR-214-5p, miR-7144-3p, miR-9830-5p/INHBA | mediated Inhibin–Activin balance | [17] | |
| GCs | circSLC41A1 | miR-9820-5p/SRSF1 | resists porcine granulosa cell apoptosis and follicular atresia | [18] | |
| GCs | circ-ANKHD1 | miR-27a-3p/SFRP1 | decreased the cell apoptosis rates | [47] | |
| Bovine | GCs | circ_n/a_75 | miR-339a | growth factor response | [15] |
| circ_n/a_303 | miR-2400 and miR-30c | [15] | |||
| Goat | follicles | chi_circ_0008219 | miR-34c-5p, miR-483, miR-1468-3p | higher fecundity rate | [23] |
| Mouse | GCs | circEGFR | miR-125a-3p/CYP19A1 | promoted granulosa cell apoptosis | [10] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms232315204
References
- Bachmayr-Heyda, A.; Reiner, A.T.; Auer, K.; Sukhbaatar, N.; Aust, S.; Bachleitner-Hofmann, T.; Mesteri, I.; Grunt, T.W.; Zeillinger, R.; Pils, D. Correlation of circular RNA abundance with proliferation–exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis and normal human tissues. Sci. Rep. 2015, 5, 8057.
- Ning, L.; Long, B.; Zhang, W.; Yu, M.; Wang, S.; Cao, D.; Yang, J.; Shen, K.; Huang, Y.; Lang, J. Circular RNA profiling reveals circEXOC6B and circN4BP2L2 as novel prognostic biomarkers in epithelial ovarian cancer. Int. J. Oncol. 2018, 53, 2637–2646.
- Teng, F.; Xu, J.; Zhang, M.; Liu, S.; Gu, Y.; Zhang, M.; Wang, X.; Ni, J.; Qian, B.; Shen, R. Comprehensive circular RNA expression profiles and the tumor-suppressive function of circHIPK3 in ovarian cancer. Int. J. Biochem. Cell Biol. 2019, 112, 8–17.
- Gao, Y.; Zhang, C.; Liu, Y.; Wang, M. Circular RNA profiling reveals circRNA1656 as a novel biomarker in high grade serous ovarian cancer. Biosci. Trends 2019, 13, 204–211.
- Zhao, Z.; Ji, M.; Wang, Q.; He, N.; Li, Y. Circular RNA Cdr1as upregulates SCAI to suppress cisplatin resistance in ovarian cancer via miR-1270 suppression. Mol. Ther.-Nucleic Acids 2019, 18, 24–33.
- Che, Q.; Liu, M.; Xu, J.; Liu, Y.; Cao, X.; Dong, X.; Liu, S. Characterization of circular RNA expression profiles in cumulus cells from patients with polycystic ovary syndrome. Fertil. Steril. 2019, 111, 1243–1251.e1241.
- Li, M.; Zeng, Z.; Zhang, A.; Ye, Q.; Su, S.; Xia, T. WGCNA analysis identifies polycystic ovary syndrome-associated circular RNAs that interact with RNA-binding proteins and sponge miRNAs. Int. J. Gen. Med. 2021, 14, 8737.
- Wang, L.-P.; Peng, X.-Y.; Lv, X.-Q.; Liu, L.; Li, X.-L.; He, X.; Lv, F.; Pan, Y.; Wang, L.; Liu, K.-F. High throughput circRNAs sequencing profile of follicle fluid exosomes of polycystic ovary syndrome patients. J. Cell. Physiol. 2019, 234, 15537–15547.
- Cai, H.; Li, Y.; Li, H.; Niringiyumukiza, J.D.; Zhang, M.; Chen, L.; Chen, G.; Xiang, W. Identification and characterization of human ovary-derived circular RNAs and their potential roles in ovarian aging. Aging 2018, 10, 2511.
- Jia, W.; Xu, B.; Wu, J. Circular RNA expression profiles of mouse ovaries during postnatal development and the function of circular RNA epidermal growth factor receptor in granulosa cells. Metabolism 2018, 85, 192–204.
- Liang, G.; Yang, Y.; Niu, G.; Tang, Z.; Li, K. Genome-wide profiling of Sus scrofa circular RNAs across nine organs and three developmental stages. DNA Res. 2017, 24, 523–535.
- Pan, X.; Gong, W.; He, Y.; Li, N.; Yuan, X. Ovary-derived circular RNAs profile analysis during the onset of puberty in gilts. BMC Genom. 2021, 22, 445.
- Liu, Y.; Zhou, Z.; He, X.; Jiang, Y.; Ouyang, Y.; Hong, Q.; Chu, M. Differentially Expressed Circular RNA Profile Signatures Identified in Prolificacy Trait of Yunshang Black Goat Ovary at Estrus Cycle. Front. Physiol. 2022, 13, 576.
- Xu, L.; Liu, C.; Na, R.; Zhang, W.; He, Y.; Yuan, Y.; Zhang, H.; Han, Y.; Zeng, Y.; Si, W. Genetic Basis of Follicle Development in Dazu Black Goat by Whole-Transcriptome Sequencing. Animals 2021, 11, 3536.
- Fu, Y.; Jiang, H.; Liu, J.B.; Sun, X.L.; Zhang, Z.; Li, S.; Gao, Y.; Yuan, B.; Zhang, J.B. Genome-wide analysis of circular RNAs in bovine cumulus cells treated with BMP15 and GDF9. Sci. Rep. 2018, 8, 7944.
- Guo, T.; Zhang, J.; Yao, W.; Du, X.; Pan, Z. CircINHA resists granulosa cell apoptosis by upregulating CTGF as a ceRNA of miR-10a-5p in pig ovarian follicles. Biochim. Biophys. Acta 2019, 1862, 194420.
- Ma, M.; Wang, H.; Zhang, Y.; Zhang, J.; Liu, J.; Pan, Z. circRNA-Mediated Inhibin–Activin Balance Regulation in Ovarian Granulosa Cell Apoptosis and Follicular Atresia. Int. J. Mol. Sci. 2021, 22, 9113.
- Wang, H.; Zhang, Y.; Zhang, J.; Du, X.; Pan, Z. circSLC41A1 Resists Porcine Granulosa Cell Apoptosis and Follicular Atresia by Promoting SRSF1 through miR-9820-5p Sponging. Int. J. Mol. Sci. 2022, 23, 1509.
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50.
- Meng, L.; Teerds, K.; Tao, J.; Wei, H.; Jaklofsky, M.; Zhao, Z.; Liang, Y.; Li, L.; Wang, C.C.; Zhang, S. Characteristics of Circular RNA Expression Profiles of Porcine Granulosa Cells in Healthy and Atretic Antral Follicles. Int. J. Mol. Sci. 2020, 21, 5217.
- Xu, G.; Zhang, H.; Li, X.; Hu, J.; Sun, S. Genome-Wide Differential Expression Profiling of Ovarian circRNAs Associated With Litter Size in Pigs. Front. Genet. 2019, 10, 1010.
- Liang, G.; Yan, J.; Guo, J.; Tang, Z. Identification of Ovarian Circular RNAs and Differential Expression Analysis between MeiShan and Large White Pigs. Animals 2020, 10, 1114.
- Hu, T.; Qi, X.; Feng, Z.; Zhang, N.; Yang, L.; Suo, X.; Li, X.; Yang, Q.; Chen, M. Circular RNA profiling reveals chi_circ_0008219 function as microRNA sponges in pre-ovulatory ovarian follicles of goats (Capra hircus). Genomics 2018, 110, 257–266.
- Liu, A.; Chen, X.; Liu, M.; Zhang, L.; Ma, X.; Tian, S. Differential expression and functional analysis of CircRNA in the ovaries of low and high fecundity hanper sheep. Animals 2021, 11, 1863.
- Li, Y.; Xia, G.; Tan, Y.; Shuai, J. Expression profile of circular RNAs in continuous light-induced ovarian dysfunction. Ecotoxicol. Environ. Saf. 2022, 242, 113861.
- Hu, J.; Wang, L.; Chen, J.; Gao, H.; Zhao, W.; Huang, Y.; Jiang, T.; Zhou, J.; Chen, Y. The circular RNA circ-ITCH suppresses ovarian carcinoma progression through targeting miR-145/RASA1 signaling. Biochem. Biophys. Res. Commun. 2018, 505, 222–228.
- Zhang, M.; Xia, B.; Xu, Y.; Zhang, Y.; Xu, J.; Lou, G. Circular RNA (hsa_circ_0051240) promotes cell proliferation, migration and invasion in ovarian cancer through miR-637/KLK4 axis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1224–1233.
- Xie, J.; Wang, S.; Li, G.; Zhao, X.; Jiang, F.; Liu, J.; Tan, W. circEPSTI1 regulates ovarian cancer progression via decoying miR-942. J. Cell. Mol. Med. 2019, 23, 3597–3602.
- Chen, H.; Mao, M.; Jiang, J.; Zhu, D.; Li, P. Circular RNA CDR1as acts as a sponge of miR-135b-5p to suppress ovarian cancer progression. Onco Targets Ther. 2019, 12, 3869.
- Ahmed, I.; Karedath, T.; Andrews, S.S.; Al, I.K.; Mohamoud, Y.A.; Querleu, D.; Rafii, A.; Malek, J.A. Altered expression pattern of circular RNAs in primary and metastatic sites of epithelial ovarian carcinoma. Oncotarget 2016, 7, 36366.
- Zhang, M.; Sun, Y.; Xu, H.; Shi, Y.; Shen, R.; Teng, F.; Xu, J.; Jia, X. Circular RNA Hsa_Circ_0007444 Inhibits Tumor Progression Through MiR-23a-3p/DICER1 in Ovarian Cancer. Res. Sq. 2021.
- Sun, S.; Fang, H. Curcumin inhibits ovarian cancer progression by regulating circ-PLEKHM3/miR-320a/SMG1 axis. J. Ovarian Res. 2021, 14, 158.
- Lin, X.; Chen, Y.; Ye, X.; Xia, X. Circular RNA ABCB10 promotes cell proliferation and invasion, but inhibits apoptosis via regulating themicroRNA1271mediated Capn4/Wnt/βcatenin signaling pathway in epithelial ovarian cancer. Mol. Med. Rep. 2021, 23, 387.
- Li, Q.-H.; Liu, Y.; Chen, S.; Zong, Z.-h.; Du, Y.-P.; Sheng, X.-J.; Zhao, Y. circ-CSPP1 promotes proliferation, invasion and migration of ovarian cancer cells by acting as a miR-1236-3p sponge. Biomed. Pharmacother. 2019, 114, 108832.
- Bao, L.; Zhong, J.; Pang, L. Upregulation of Circular RNA VPS13C-has-circ-001567 Promotes Ovarian Cancer Cell Proliferation and Invasion. Cancer Biother. Radiopharm. 2019, 34, 110–118.
- Zhao, Y.; Qin, X.P.; Lang, Y.P.; Kou, D.; Shao, Z.W. Circular RNA circ-SMAD7 promoted ovarian cancer cell proliferation and metastasis by suppressing KLF6. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5603–5610.
- Hou, W.; Zhang, Y. Circ_0025033 promotes the progression of ovarian cancer by activating the expression of LSM4 via targeting miR-184. Pathol.—Res. Pract. 2021, 217, 153275.
- Zhao, C.; Zhou, Y.; Shen, X.; Gong, M.; Lu, Y.; Fang, C.; Chen, J.; Ju, R. Circular RNA expression profiling in the fetal side of placenta from maternal polycystic ovary syndrome and circ_0023942 inhibits the proliferation of human ovarian granulosa cell. Arch. Gynecol. Obstet. 2020, 301, 963–971.
- Chen, A.-X.; Jin, R.-Y.; Zhou, W.-M.; Ye, Y.-J.; Lu, J.-L.; Ren, Y.-F.; Xuan, F.-L. CircRNA circ_0043533 facilitates cell growth in polycystic ovary syndrome by targeting miR-1179. Reprod. Biol. 2022, 22, 100637.
- Lu, X.; Gao, H.; Zhu, B.; Lin, G. Circular RNA circ_RANBP9 exacerbates polycystic ovary syndrome via microRNA-136-5p/XIAP axis. Bioengineered 2021, 12, 6748–6758.
- Wu, G.; Xia, J.; Yang, Z.; Chen, Y.; Jiang, W.; Yin, T.; Yang, J. CircASPH promotes KGN cells proliferation through miR-375/MAP2K6 axis in Polycystic Ovary Syndrome. J. Cell. Mol. Med. 2022, 26, 1817–1825.
- Zhang, D.; Yi, S.; Cai, B.; Wang, Z.; Chen, M.; Zheng, Z.; Zhou, C. Involvement of ferroptosis in the granulosa cells proliferation of PCOS through the circRHBG/miR-515/SLC7A11 axis. Ann. Transl. Med. 2021, 9, 1348.
- Tu, P.; Yan, S.; Zhang, F. Circ_0005925 Promotes Granulosa Cell Growth by Targeting MiR-324-3p to Upregulate MAP2K6 in Polycystic Ovary Syndrome. Biochem. Genet. 2022, 1–14.
- Xu, L.; Xiong, F.; Bai, Y.; Xiao, J.; Zhang, Y.; Chen, J.; Li, Q. Circ_0043532 regulates miR-182/SGK3 axis to promote granulosa cell progression in polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2021, 19, 103681.
- Zhou, J.; Zhou, J.; Li, Y.-Y.; Li, M.-Q.; Liao, H.-Q. CircRNA circUSP36 impairs the stability of NEDD4L mRNA through recruiting PTBP1 to enhance ULK1-mediated autophagic granulosa cell death. J. Reprod. Immunol. 2022, 153, 103681.
- Cai, H.; Chang, T.; Li, Y.; Jia, Y.; Li, H.; Zhang, M.; Su, P.; Zhang, L.; Xiang, W. Circular DDX10 is associated with ovarian function and assisted reproductive technology outcomes through modulating the proliferation and steroidogenesis of granulosa cells. Aging 2021, 13, 9592.
- Li, X.; Gao, F.; Fan, Y.; Xie, S.; Li, C.; Meng, L.; Li, L.; Zhang, S.; Wei, H. A novel identified circ-ANKHD1 targets the miR-27a-3p/SFRP1 signaling pathway and modulates the apoptosis of granulosa cells. Environ. Sci. Pollut. Res. 2021, 28, 57459–57469.
This entry is offline, you can click here to edit this entry!
