1. Early Prediction of Pathological Outcome after NACT
Early prediction of pCR following NACT could be an important tool for personalized medicine, allowing the selection of patients eligible for NACT [
53].
Many studies have demonstrated how the evaluation of multiparametric breast MRI obtained before the start of NACT can predict which cancer will achieve pCR, through the analysis of morphological and functional features and the application of advanced imaging and artificial intelligence (AI) techniques.
Thus, the challenge remains the early and accurate predictions of NACT response.
1.1. Morphological Characteristics
Among the morphologic MRI features, an expansively growing carcinoma forming a relatively well-defined round/oval or lobulated mass is often associated with low rates of pCR, suggesting that careful attention should be paid during the course of the therapy in these types of tumors [
54]. In patients with HER2 positive or TN breast cancer, one recent study identified an orientation of growth parallel to Cooper’s ligaments as an accurate predictor of pCR [
55]. Indeed, the growth pattern may be a sign of a tumor’s microarchitecture and influence on its micro-environment: tumors growing more slowly can cause desmoplastic reaction and grow crossing Cooper’s ligaments, while tumors with faster and more expansive growth will propagate parallel to Cooper’s ligaments [
55].
In a study by Thompson et al. [
56], the authors found a significant association between tumor spread at baseline MRI and pCR, with multicentric tumors being associated with a lower possibility of pCR compared to multifocal or single lesion. Likewise, multifocal multicentric lesions and non-mass enhancement in pre-treatment MRI were significantly associated with absence of pCR in a multivariate analysis of radiological findings by Choi et al. [
57].
1.2. Background Parenchyma Enhancement (BPE)
Some authors have suggested a correlation between the background parenchymal enhancement (BPE) and tumor response after NACT. BPE is defined as normal enhancement of the fibro-glandular tissue on contrast-enhanced (CE) MRI and is thought to be associated with poorer prognosis: several studies found a significant association between high BPE on baseline MRI and worse recurrence free survival (RFS) [
58,
59]. This is probably due to higher perfusion into the breast, leading to higher BPE, and proangiogenic breast ecosystem for tumor growth or higher amounts of physiologically active breast tissue more susceptible to tumor transformation [
60]. Nevertheless, evaluation of the BPE of the tissue surrounding the tumor can be difficult and relies on the selection of a proper image and the placement of the region of interest.
In a study by Preibsch H et al. [
61], the authors found that the reduction of BPE on CE-MRI in patients undergoing NACT may predict tumor response. Since CE-MRI is performed bilaterally, some authors measured the BPE of the contralateral healthy breast and found no associations between BPE at pre-treatment MRI and response to NACT [
62,
63]. Thus, suggesting that other factors such as Ki-67 or menopausal status play a more important prognostic role.
1.3. Diffusion Weighted Imaging (DWI) and Apparent Diffusion Coefficient (ADC)
Functional imaging sequences, such as DWI-MRI and CE-MRI, allow the analysis of biological characteristics of tumors, such as cellularity and neo-angiogenesis, by assessing cellular density of a tissue, quantified using ADC map. However, the analysis of the ADC value on pretreatment and pCR imaging remains controversial, since some tumors may have low cellularity (and high ADC value) due to local necrosis secondary to hypoxia or local fibrosis or desmoplastic components [
64]. According to some authors, a tumor with low cellularity is less likely to respond to NACT, possibly due to lack of perfusion and/or slow tumor growth that causes impaired drug administration; while highly cellular tumors (therefore with low ADC values) are more chemo-sensitive [
55,
65,
66].
In one study, the best pre-treatment ADC cut-off value to distinguish between responders and non-responders was found to be 0.55 mm
2/s based on the ROC curve analysis (area under the ROC curve was 0.65; 95% CI, 0.415–0.831) [
55]. In another study the best pretreatment ADC cutoff was 1.17 × 10
−3 mm
2/s using a high
b-value of 750 s/mm
2, with a sensitivity of 94% and a specificity of 71% [
66].
Conversely, some other authors found no significant difference in the mean ADC value between responders and non-responders in the general population [
67,
68]. However, when performing the analysis into different subgroups according to tumor phenotypes, the authors observed a statistically significant difference in the mean value of ADC between responders and non-responders in TN and HER2 positive tumors [
68,
69]. The difference in results indicate an effect modification according to the classified subtype.
Some works investigated total choline (TCho) levels in breast cancer through Magnetic Resonance Spectroscopy (MRS). TCho is a biomarker of elevated cellular turnover and is thought to be an early imaging predictor of response to treatment but its prognostic value remains unconfirmed [
70,
71]. However, the technical complexity and limited diffusion of MRS inhibit its use for response assessment in multicentric studies [
72].
1.4. Triple Negative Breast Cancer
Different cancer subtypes show different pCR rates with higher rates in HER2 positive and TN breast cancer [
73], reaching 68–80% in patients having carboplatin or dual HER2 blockade [
74].
However, TN breast cancer is more aggressive than other subtypes and associated with a higher rate of relapse and a lower rate of OS [
65,
71].
TN breast cancer is more likely to display intramammary edema, resulting from lymphovascular invasion. Peritumoral edema occurs due to local reaction of the surrounding tissue to the tumor, pre-pectoral edema is determined by marked lymphovascular invasion, whereas subcutaneous edema reflects the lymphovascular invasion. The correlation between response to NACT and the presence of edema is controversial. Bae et al. [
75] revealed that the presence of peritumoral edema is associated with low pCR, even though it isn’t significantly associated with worse RFS. However, more recent studies found no correlation between the presence of intramammary edema and response to NACT [
76,
77]. Reported edema might not imply the reporting of the predominant underlying cause (inflammation, angiogenesis, infiltration of tumor cells, tumor emboli) and this may explain the difference of the findings in the literature.
Another common finding in TN breast cancer is intratumoral necrosis, probably due to the increased mitotic activity, which is often associated with poor or no response to NACT [
78]. However, a recent study found no significant correlation between the presence of intratumoral necrosis and pCR in patients with TN breast cancer [
77]. Small numbers of patients might affect these conflicting findings.
In addition, it has been shown that TN breast cancer presenting as an irregular mass is less likely to respond to NACT than that with other appearances [
78].
In a study by Li et al. [
79], a nomogram based on baseline dynamic CE-MRI was developed to predict pCR; in particular, it was observed that time to peak (TTP), tumor volume measured on CE-MRI and androgen receptor (AR) grade were independent predictors of pCR.
1.5. What’s New (Radiomics, Machine Learning and Radiogenomics)
Radiomic analysis is a method to extract quantitative information from each voxel of radiological images, to predict clinical data. Radiomics applications are constantly increasing given their ability in the prediction of pCR in various cancer types, including breast cancer [
80]. In a retrospective study, Cain et al. [
81], developed multivariate machine learning models based on pre-treatment MRI features that were able to predict pCR in TN and HER2 positive patients. Similarly, Liu et al. [
82] developed and validated radiomics models combining T2-w imaging, DWI, and contrast-enhanced T1-w imaging on pre-treatment MRI. The radiomic signature performed relatively well in ER-positive, HER2 negative and TN groups. Braman et al. [
83] examined intratumoral and peritumoral features, since they both contribute to response predictions. Recently, Huang et al. [
84] have demonstrated that the tumor shrinkage pattern can be accurately predicted by a model that combines clinicopathologic appearances and radiomics features. These findings imply that pre-treatment breast MRI could be a means to stratify patients and address them to more appropriate treatment options, tailoring treatment and thus improving quality of life. Radiogenomics associates radiomics imaging features to tumor genetic profiles and represents an exciting field of research that might further improve the performance of predictive models [
85,
86].
2. The Role of MRI in the Preoperative Assessment of Residual Disease and Pathological Complete Response (pCR)
The definition of pCR is still the object of debate with some trials defining it as the absence of both in situ and invasive cancer while others considering only the invasive component [
87]. The accuracy of MRI in detecting residual disease after NACT differs according to pCR definition and it is higher when the resolution of invasive disease only is considered pCR [
13,
88].
pCR has been proven to be a good prognostic marker to predict long-term survival in breast cancer [
7,
89]; therefore, it is considered a suitable surrogate end point for patients with luminal B/HER2 negative, HER2 positive (non-luminal), and TN disease but not for those with luminal B/HER2 positive or luminal A tumors [
86,
90]. However, only about 30% of patients achieve pCR after NACT, and the pCR rate changes according to different molecular subtypes, as for tumor size and treatment regimen [
91].
In order to reach the maximum surgical advantage from NACT, it is crucial to correctly evaluate the tumor response and residual disease prior to surgery. The Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 guidelines [
92] recommend measurement of the longest diameter of a solid tumor in at least one dimension. Multicentric disease should be evaluated by summing the largest diameter of the detectable tumors. Although MRI can both over- and underestimate residual disease [
13], the longest diameter measured on the first contrast-enhanced phase of MRI performed after NACT better evaluate the extent of residual disease than clinical exam, mammography, and HHUS [
14,
17,
93].
2.1. Pattern of Tumor Response
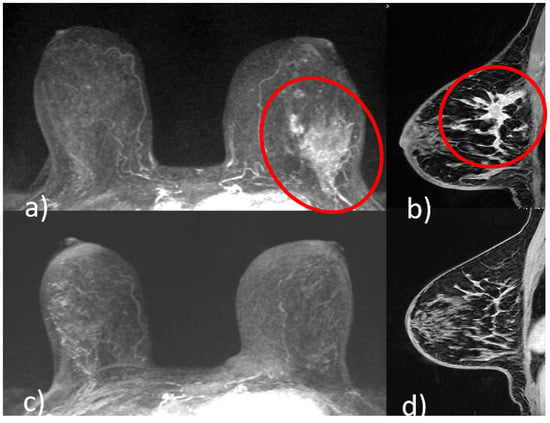
Different shrinkage patterns are seen in breast cancer after NACT, including no residual tumor (pCR) (
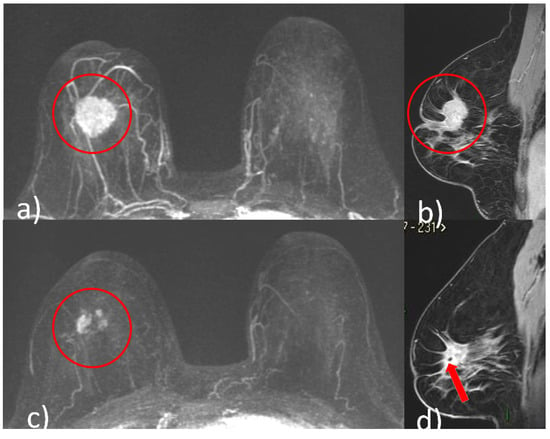
Figure 1), concentric shrinkage (i.e., only one residual invasive tumor focus, without DCIS), multifocal shrinkage (i.e., more than two invasive) (
Figure 2), diffuse shrinkage (i.e., a main residual invasive focus with surrounding satellite DCIS), stable disease (SD), and progressive disease (PD) [
84].
Figure 1. A 51-year-old patient with invasive ductal carcinoma (ER 40% PR 3% Ki67 40% HER2 3+) of the upper outer quadrant of the left breast. Pre-NACT CE-MRI revealed an area of non-mass enhancement with segmental distribution in the left breast that extends to pectoralis muscle without signs of invasion (red circle, (a) axial maximum intensity projection reconstruction image; red circle, (b), sagittal post-contrast T1-weighted image). At the end of NACT, CE-MRI showed no residual tumor (pCR) ((c) axial maximum intensity projection reconstruction image; (d) sagittal post-contrast T1-weighted image).
Figure 2. A 48-year-old patient with G2, luminal B, HER2 positive right breast cancer undergoing NACT. Pre-treatment breast CE-MRI showed an oval mass with irregular margins, nipple invasion and skin retraction at the junction of upper quadrants of the right breast (red circle, (a) axial maximum intensity projection reconstruction image) (red circle, (b) sagittal post-contrast T1-weighted image). After NACT, multifocal shrinkage was depicted by CE-MRI (red circle (c), axial maximum intensity projection reconstruction image). (d) Signal void artifact caused by tissue marker clip inside the residual mass is well visible on the sagittal T1-weighted post-contrast image (red arrow).
Kim et al. [
94] found that the extent of residual disease obtained at MRI was significantly correlated with the evaluation obtained at pathology, excluding crumbling/residual multicentric shrinkage pattern. Tumor response as multiple, scattered deposits may also make assessment of the longest diameter difficult, with different approaches to measurement that either include [
95] or exclude [
96] intervening normal tissue. Concentric shrinkage or multicentric shrinkage patterns halfway through therapy often indicate response at the end of therapy, while diffuse non-mass enhancement, stable disease, and progression are strongly related with non-response [
97]. The probability of underestimating residual disease is reported as being higher for non-mass enhancement than for masses. Reactive inflammation, fibrosis or necrosis in response to NACT may appear as areas of enhancement on MRI, that might be challenging to distinguish from residual tumor [
18,
98].
Some authors tried to quantify the degree of residual enhancement by using the lesion to background parenchymal signal enhancement ratio (SER), which has been found to increase the specificity in detecting residual tumor rather than using size criteria alone (with SER ≤ 1.6 indicating pCR) [
99].
It has been suggested that the type of chemotherapy agent should be considered when using MRI in the evaluation of tumor response. Patients treated with taxane-containing regimens [
23] and in HER2 negative patients receiving bevacizumab or paclitaxel [
24,
25], residual disease was frequently underestimated.
2.2. Impact on Therapy and surgical planning
MRI also allows in vivo evaluation of treatment efficacy, which may permit changing the treatment approach if the tumor is not responding. Some studies reported the ability of MRI to assess the efficacy of treatment early, after 1–2 cycles of NACT, based on imaging data of volumetric changes, kinetic analysis, both [
100] and metabolic changes [
101,
102]. However, the use of MRI to inform changes to the NACT regimen has been shown only in one retrospective study [
103].
Overall, based on its ability to show the extent of tumor after NACT and increasing numbers of studies demonstrating early response prediction, MRI is a useful tool in helping the multidisciplinary team to choose the most suitable therapeutic planning, especially surgical treatment aimed at local disease control. However, up to now, no randomized control trial has demonstrated that using MRI for monitoring the response to NACT increases the rate of breast conserving surgery. On the other hand, also in the neoadjuvant setting, MRI is considered useful for appropriate surgical decision making likewise to preoperative MRI in the absence of NACT [
15].
Moreover, in patients with excellent response to NACT and subsequent pCR, it is oncologically safe to de-escalate treatment [74,116,117]. Accurate tailoring of NACT allows consideration of individualized locoregional treatment strategies. Heil et al. [118] put it to the extreme: they proposed to postpone or avoid breast surgery, trying a “watch and see” strategy, in patients that showed pCR at the end of NACT. This patients are very unlikely to face distant recurrence, indeed; in contrast, minimal residual pathology with subsequent risk of local relapse is still possible. Studies are currently underway to identify pCR, using barely invasive biopsies in women who have had partial/complete radiologic response after treatment, in order to stratify low-risk patients [122].
In addition, there may also be subset of patients who do not benefit from surgery after treatment; however, there is currently insufficient scientific evidence. Therefore, several questions remain unsolved, and it remains a great challenge for the future to conduct high-quality clinical trials that can answer these questions [117].
This entry is adapted from the peer-reviewed paper 10.3390/cancers14235786