1. Definitions and Classification of Electrochemical Biosensors
An electrochemical biosensor is a self-contained integrated device that can provide specific quantitative or semi-quantitative analytical information of molecular recognition events into an analytically valuable signal using a biological recognition element (bioreceptor) that is retained in direct spatial contact with an electrochemical transduction element [
47,
48]. The main function of the transducer is to convert a molecular biorecognition event into a measurable signal proportional to the analyte concentration [
49]. According to the type of bioreceptor, there are biosensors based mainly on whole cells, enzymes, antigens, antibodies, nucleic acids, aptamers, lectins, and glycans [
49,
50,
51]. The bioreceptor is the element that confers specificity and selectivity to the biosensor, which are relevant characteristics of these devices, and refers to the ability to detect a specific analyte in a mixture that contains interferences [
50] and differentiate the target from homologous counterparts.
Other characteristics of biosensors are sensitivity, linear range, reproducibility, and stability. Sensitivity is the slope of the calibration curve and is related to the limit of detection (LOD). The LOD is the lowest concentration of an analyte in a sample that can be detected, with reasonable certainty, for a given analytical procedure [
63]. The linear range is the concentration range over which the signal output is directly proportional to the concentration of the analyte and is often correlated with a straight line [
64]. Reproducibility is the closeness and agreement between independent results obtained with the same method on identical test material but under different conditions (different operators, apparatus, laboratories, or time intervals) [
63]. Finally, stability is the degree of biosensor susceptibility to ambient disturbances. One way to assess the stability is by continuously or sequentially performing biosensor exposure to analyte solution or by measuring the change in the baseline or sensitivity over a fixed period [
65,
66]. These characteristics make electrochemical biosensors affordable, accurate, rapid, and sensitive analytical platforms for detecting multiple disease biomarkers [
67].
In summary, electroanalytical methods allow the precise determination of multiple analytes with high sensitivity and fast response. Table 1 helps sort out electrochemical glycobiosensors by comparing electroanalytical methods based on analytical performance and the detection principle.
Table 1. Table of electrochemical glycobiosensors and analytical performance.
| Electrochemical Technique |
Sensing Principle |
The Typical Range of the Limit of Detection (LOD) |
Reference |
| CV |
Application of a time-dependent potential to an electrochemical cell and measuring the resultant current as a function of the applied potential |
10−6–10−15 M |
[69] |
| DPV |
| SWV |
| Amperometry |
| Potentiometry |
Perturbation of the potential of the electrochemical cell |
10−3–10−6 M |
[59] |
| Conductometry |
Quantification of the conductance change in the electrochemical cell |
| EIS |
Application of a small sinusoidal voltage perturbation in a range of frequencies while monitoring the resulting current |
10−9–10−18 M |
[22] |
| ECS |
2. Characterization of Electrochemical Glycobiosensors
The electrode surface of electrochemical glycobiosensors is usually characterized in terms of surface chemistry, morphology, and electrochemical performance. Atomic force microscopy (AFM) is a technique used to characterize the electrode surface morphology in glycobiosensors; it can operate in multiple modes, such as electrochemical AFM, which enables the analysis of electrochemical reactions occurring at the electrode [
70]. Morphology and surface chemical composition are characterized via scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDX) [
71,
72]. Furthermore, infrared (FT-IR) and X-ray photoelectron spectroscopic (XPS) techniques are used to analyze surface chemical composition [
73]. In addition, thermogravimetric analysis (TGA) is applied to analyze the materials used to modify the electrode surface and implies studying sample mass change under programmed conditions. Therefore, TGA is mainly used to analyze certain thermal events, such as absorption, adsorption, desorption, vaporization, sublimation, decomposition, oxidation, and reduction [
74].
Similarly, differential scanning calorimetry (DSC) measures the amount of energy absorbed or released by the sample when it is heated or cooled. TGA is a versatile technique used to study the self-assembly of supramolecular nanostructures such as glycopolymers, latent heat of melting, denaturalization temperatures, and compositional analysis [
75]. On the other hand, the optical properties are mainly characterized via ultraviolet-visible (UV-vis) spectroscopy and photoluminescence (PL) techniques [
76]. Finally, electrochemical performance is characterized via voltammetric techniques such as CV and DPV and spectroscopic techniques such as EIS and ECS [
77,
78], as commented in the description section. The affinity constants of glycan-based biorecognition elements can be determined using the Biacore system [
79]. Biacore uses surface plasmon resonance (SPR) as a label-free detection technique to monitor the interaction between biomolecules in real-time. The biorecognition molecule is immobilized on a sensor chip’s surface. At the same time, the sample containing its ligand is injected over the surface at a constant flow rate through a microfluidic channel system. The changes in mass concentrations at the surface of the sensor chip due to molecule association/dissociation are measured as an SPR response and displayed as a time function [
80].
Table 2 shows the most common characterization techniques of electrochemical glycobiosensors.
Table 2. Characterization techniques of electrochemical glycobiosensors.
| Characterization Technique |
Properties |
Technique Principle |
References |
| AFM |
Morphology |
Measurement of intermolecular forces and “seeing” atoms by using probe surfaces. |
[70] |
| SEM/EDX |
Morphology
Composition |
Application of kinetic energy to produce signals from the interaction of the electrons (secondary, backscattered, and diffracted backscattered). Secondary and backscattered electrons are used to visualize the morphology, and backscattered are related to composition. |
[71,72] |
| FT-IR |
Surface chemical composition |
Measurement of the vibrations of atoms, and from this, functional groups are determined. |
[73] |
| XPS |
Surface chemical composition |
The sample is irradiated with an X-ray, and some electrons become excited enough to escape from the atoms. The photo-ejected electrons are collected by an electron analyzer that measures their kinetic energy, allowing the element to be identified. |
[73] |
| TGA, DSC |
Sorption Composition |
The sample is heated or cooled under controlled conditions and changes in some physical properties are measured. |
[74,75] |
| UV-vis, PL |
Optical |
Light absorption and scattering by a sample. |
[76] |
| CV, DPV, EIS, ECS |
Electron transfer kinetics |
Perturbation of the electrode by applying an electric potential and recording the resulting current. |
[77,78] |
| Biacore |
Bioreceptors affinity |
The change in SPR response is measured after association/dissociation of a bioreceptor and ligand, respectively, with the sample flow in a microfluidic channel. |
[80] |
3. Nanostructured Electrochemical Glycobiosensors
Nanostructured biosensors are analytical devices that integrate nano- and bio-materials platforms for trace detection of biomolecules or chemical analytes [
81]. Due to their size-dependent properties, such as a large surface area with improved conductivity and reactivity, nanomaterials are used for developing highly sensitive biosensors [
82]. As a result, a wide range of nanomaterials has been incorporated onto the electrode surface to improve the biosensor analytical performance, such as carbon-based nanomaterials, noble metals, metal oxides, metal chalcogenides, magnetic nanoparticles, and conductive polymers, among others [
83].
Likewise, nanomaterials are similar in size to most biological entities such as proteins, nucleic acids, lipids, cells, viruses, glycans, etc., making them ideal interfaces between these entities and signal transduction surfaces as those used in biosensors [
84,
85,
86,
87]. Furthermore, stability, biocompatibility, and the advantage of modulating the nanomaterial’s surface chemistry make them suitable for conjugating multiple chemical species and biomolecules [
83]. One general advantage of all nanomaterials is the high specific surface area that enables a high surface loading of biorecognition elements on the electrode surface and their resultant improved electron transfer and electrocatalytic activity ability [
83,
88]. Their combination with suitable bioreceptors such as glycans could originate synergistic effects eliciting unforeseen benefits [
89]. Therefore, an essential issue for nanobiosensor development is the size, structure, chemical composition, shape, and nanomaterial’s surface modification [
87].
3.1. Synthesis of Nanomaterials and Surface Biofunctionalization
There are different methods to synthesize nanomaterials depending on their type and nature. In summary, the two main methods to synthesize nanomaterials are “top-down” and “bottom-up” approaches [
88]. In the top-down approach, the nanomaterial synthesis uses the size reduction from bulk materials down to the nanoscale. Unlike the top-down method, the bottom-up synthesis of nanomaterials consists of obtaining nanostructures from elementary-level building blocks of atomic or molecular size [
88]. The most common methods used to synthesize nanomaterials are the chemical vapor deposition method, thermal decomposition, hydrothermal synthesis, solvothermal method, pulsed laser ablation, templating method, combustion method, microwave synthesis, gas phase method, and conventional sol–gel method [
88].
As mentioned above, glycan-based biorecognition elements confer specificity and selectivity to glycobiosensor devices, recognizing the target analyte and binding it to the sensor surface for transduction [
66]. Nanomaterials are supporting platforms for glycan-based bioreceptors attachment by physical and chemical methods [
90] and enhancing analytical performance [
91]. Such bioreceptors are immobilized to the nanomaterials by physical methods without chemical bond formation through physical entrapment, microencapsulation, adsorption, and sol–gel techniques [
92]. Unlike physical methods, chemical approaches form covalent bonds in the presence of two mutually reactive chemical groups from the bioreceptors and the substrate surface [
90,
93]. One of the more common approaches involves amide bond formation in the presence of carboxylic acids (-COOH) and primary amines (-NH
2). This approach requires activation of -COOH with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) or sulfo-NHS, producing esters, which react with primary amines to form amides. Another approach couples the bioreceptor via the free thiols (-SH), which react stoichiometrically with maleimides through a Michael addition reaction [
93]. The glycans also can be functionalized with thiols and disulfide-bearing linkers for the direct formation of self-assembled monolayers (SAMs) on metallic surfaces, i.e., gold. Alternatively, some linkers can be pre-assembled on the transducer surface for subsequent attachment of glycans via reactive terminal groups [
94,
95].
Bioreceptors, like glycans, are also immobilized onto conducting polymers with redox properties, such as polystyrene sulfonate, polyvinyl ferrocene, polythiophene, polyaniline, and quinone polymers, which results in an enhancement of the biosensor electrochemical performance [
96]. Furthermore, there are immobilization strategies based on affinity interactions, such as the biotin–avidin interaction or immobilizing binding proteins such as Protein A or G onto the electrode surface, followed by the subsequent capture of antibodies and blocking of the nonspecific adsorption sites steps with bovine serum albumin (BSA), casein, or other blocking agents [
90,
93,
97,
98,
99,
100].
3.2. Operation Modes of Electrochemical Nanobiosensors
Electrochemical biosensors that integrate nanostructures and suitable biorecognition elements on the electrode surface are highly sensitive and specific. These features enable the detection and quantification of disease biomarkers at ultra-low concentrations, which is a requisite for the early diagnosis of diseases [
88,
101]. On the other hand, regarding the detection of molecular biorecognition events on the electrode surfaces, various labeling strategies are used to amplify the detection signal on electrochemical biosensors. Label-based approaches can involve avidin–biotin conjugation with redox enzymes, covalent attachment, intercalation, or electrostatic interaction of small molecules, particles, or ions with the biorecognition elements responsible for generating the electrochemical signal. In contrast, label-free biosensors directly transduce a molecular binding event into a physically measurable quantity, i.e., without needing an additional antibody, enzymatic, fluorescent, or electroactive label, or any other amplification strategy, to provide a response that is proportional to the concentration of bound molecules [
102]. Label-free electrochemical biosensors measure interfacial electrical property changes, such as charge transfer resistance or electrochemical capacitance, through the EIS or the ECS techniques and by measuring current changes via CV, DPV, or SWV [
22,
77].
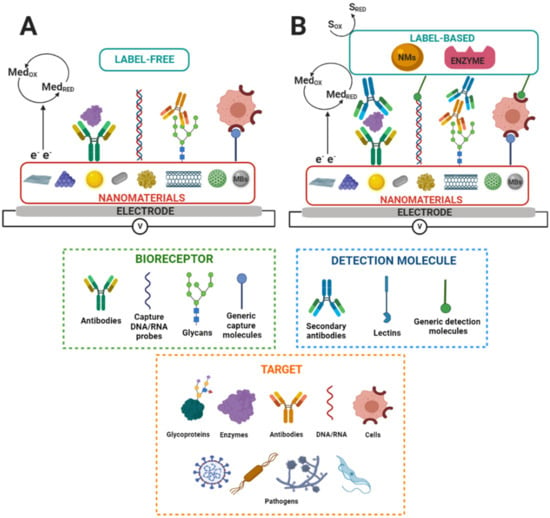
Figure 1 shows a label-free and label-based nanobiosensors setup.
In summary, electrochemical nanobiosensors based on glycans offer exceptional attributes for disease diagnosis, such as being affordable, sensitive, specific, us
Figure 1. Scheme of nanostructured electrochemical biosensors. S
RED and S
OX and Med
RED and Med
OX indicate substrate reduction and oxidation and mediator reduction and oxidation, respectively. MBs indicate magnetic beads. (
A) Label-free and (
B) label-based nanobiosensors set up. Adapted from [
83].
er-friendly, portable, rapid, robust, simple to construct, equipment-free, and deliverable to all people in need [
2,
103]. In addition, nanostructured electrodes provide a large surface area to immobilize a high load of bioreceptors on the electrode surface and enhance the electron transfer and electrocatalytic activity ability, resulting in biosensors of enhanced analytical performance [
82].
3.3. Glycans as Biorecognition Elements in Electrochemical Biosensors
A myriad of bioreceptors, such as antibodies, nucleic acids, aptamers, peptides, enzymes, etc., have been used in biosensors as biorecognition elements for detecting multiple biomarkers [
68,
104,
105,
106]. However, the application of glycans as bioreceptors is less explored in biosensor platforms to develop new diagnosis/prognosis tests [
107].
The most common types of electrochemical glycobiosensors use lectins as selective biorecognition elements. Lectins are natural proteins that recognize and reversibly bind to specific free carbohydrates and terminal groups on glycans of glycoconjugates [
59,
67,
108,
109]. Lectin-based biosensors have been used for detecting CEA, P-glycoprotein, prostate-specific antigen (PSA), and viruses [
67,
110,
111].
On the other hand, there are some applications where glycans are attached to the electrode surface as biorecognition elements [
62]. In two of these applications, a glycan sialyllactose was immobilized on SAM-modified gold electrodes via amine coupling to detect proteins present on the envelope of influenza viruses [
112,
113]. Similarly, another application uses a mannose glycan immobilized on SAM-modified gold electrodes for bacteria detection [
114]. Furthermore, there is a report for the immobilization of Tn antigen (N-galactosamine attached to serine) on SAM-modified gold electrodes for binding a tumor-associated antibody [
115]. In addition, there are reports describing glycans immobilization with a built-in redox center. In these works, glycans were attached to quinone moieties and applied to detect intact bacterial and cancerous cells using graphene-modified electrodes [
116,
117,
118].
In summary, glycan immobilization on the electrode surface depends on the glycan structure and the electrode surface chemistry. Glycans specifically recognize molecular targets and confine them on the electrode surface. The biorecognition molecular event changes the interfacial electrical properties, and the analyte concentration correlates with the changes in electrical properties measured by electrochemical techniques.
The bioreceptor affinity is inversely related to the dissociation constant K
D. It describes the binding strength between a bioreceptor, such as a lectin or an antibody, and its ligand [
119]. K
D is in the nM–mM range for lectin–glycan interactions and pM-nM for oligonucleotide hybridization and antibody–antigen interactions [
120,
121,
122,
123]. Lectins have multivalence to recognize glycans, allowing significant affinity amplification and even reaching the subnanomolar range [
121]. In addition, engineered glycomaterials allow for overcoming affinity and selectivity challenges in glycan-based molecular binding events achieving affinity in the picomolar range [
124]. For this reason, glycans are promising bioreceptors for electrochemical biosensors, as mentioned.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27238533