Extracellular vesicles (EVs) form a heterogeneous group of membrane-enclosed structures secreted by all cell types. EVs export encapsulated materials composed of proteins, lipids, and nucleic acids, making them a key mediator in cell–cell communication. In the context of the neurovascular unit (NVU), a tightly interacting multicellular brain complex, EVs play a role in intercellular communication and in maintaining NVU functionality. In addition, NVU-derived EVs can also impact peripheral tissues by crossing the blood–brain barrier (BBB) to reach the blood stream. As such, EVs have been shown to be involved in the physiopathology of numerous neurological diseases.
- extracellular vesicles
- NVU
- neuroinflammation
1. Introduction
2. EVs in the Central Nervous System
2.1. Neurovascular Unit Structure
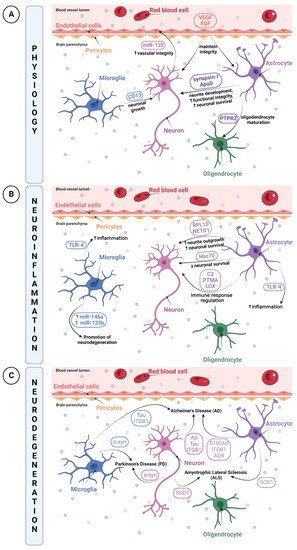
The Neurovascular Unit (NVU) is a relatively new concept describing the relationship between brain cells with their environment and blood vessels. Knowing the NVU structure is essential to better understand brain physiopathology. The NVU is a multicellular complex composed of vascular cells such as endothelial cells, pericytes, and vascular smooth muscle cells, as well as glial cells including astrocytes, microglia and oligodendrocytes, and lastly neurons. All of these cell types are interdependent and act in concert with various physiological processes of the brain [8][9][10]. Under normal physiological conditions, NVU cells interaction results in a highly efficient barrier, whose main functions are to regulate the brain’s homeostasis, maintain its integrity, and protect it against insults (i.e., infections, trauma, and inflammation) [11]. A pivotal part of this complex cellular unit appears to be its ability to maintain brain integrity through junctional complexes, allowing them to be intimately and reciprocally linked to each other [11].
2.2. Roles of EVs in the NVU
It has been reported that all cell types of the NVU release EVs [3][25]. An important characteristic of EVs is that they can cross the BBB to reach peripheral blood and later, other organs. EV transport is mostly mediated by a receptor-based mechanism and transcytosis [26][27]. By crossing this determinant barrier, EVs become an attractive tool for the diagnosis, prognosis or treatment of certain diseases. They can be a rich molecular biomarker source, a cell therapy surrogate or a drug delivery vehicle [28]. However, knowledge of the physiological role of EVs in the CNS is still limited [1][29][30].
2.2.1. Roles of NVU Cell-Derived EVs in Physiological Conditions
2.2.2. Roles of NVU Cell-Derived EVs in Pathological Conditions
Neuroinflammation
Neuroinflammation is a biological process by which the innate immune system of the brain is activated after an inflammatory event such as an infection, toxin exposure, a neurodegenerative disease, aging or brain traumas [41][42][43][44]. This triggers an immediate and short activation of the innate immune system, mainly characterized by the release of inflammatory mediators such as cytokines and chemokines, and by increased BBB permeability [45]. However, a prolonged and amplified inflammatory response may have a detrimental impact due to excitotoxicity or oxidative stress, resulting in BBB breakdown [46]. Those processes can cause further damage to the surrounding tissue of the initial neurovascular injury, leading to secondary brain injuries [47][48][49]. Moreover, the pro-inflammatory microenvironment created by activated microglia and astrocytes and their release of cytokines and chemokines can increase tissue injury [50]. Red blood cells lysis and excess thrombin also produce cytotoxicity, enhancing brain damage and BBB disruption [47][48][49].
Thus, when neuroinflammation occurs, particular inflammatory mediators may be transported by EVs, notably by microglia that are regarded as resident immune cells of the CNS, to communicate the current inflammatory state. It has been reported that EVs from microglia had upregulated expressions of miR-146a and miR-125b, involved in the regulation of the NF-κB pathway as well as in microglial activation, revealing EVs as promising modulators by promoting neuroregeneration [51]. In parallel, the study of Kumar et al. demonstrated that after a trauma, EVs released from microglia, which were initially loaded with proinflammatory molecules, were able to activate other microglia. This contributes to the ongoing neuroinflammatory reply in the injured brain and to the activation of immune responses [52].
Although, under multiple stimuli, EVs transport immune response elements, they are also able to propagate inflammatory mediators during diseases or disorders. Indeed, TLR-4 expression in EVs was already shown to be increased, enhancing cytokines and ROS production in EVs from microglia and astrocytes. This increase resulted in a transmission of inflammation via EVs, which provides evidence in using EVs as biomarker cargos [53][54].
Moreover, it is widely recognized that coagulopathy is an important factor for secondary brain injury in trauma patients, which is related to poor outcome and may be associated with neuroinflammation and enhanced BBB permeability [55]. Indeed, there is strong evidence of a reciprocal activation between inflammation and coagulation, mainly mediated by the tissue factor pathway [56]. Regarding EVs, several studies on animals and humans have shown that platelets and cell-derived EVs could have procoagulant action [57][58][59][60], which relies on the exposure of phosphatidylserine (PS) on their surface and/or tissue factors (TF), the primary initiator of coagulation in vivo [57][61][62]. Under pathological conditions, coagulant TF-exposing microparticles can directly initiate coagulation and thrombus formation by being recruited to sites of vascular injury in vivo [62][63]. In addition, EVs, upon exposure to negatively charged phospholipids such as PS, provide a catalytic platform supporting coagulation through the facilitated formation of tenase and prothrombinase complexes [57][64].
Taken together, studies on brain-derived EVs demonstrated that they do not simply mediate the inflammatory response. Indeed, EVs associated with proinflammatory and procoagulant molecules were reported to trigger different biological processes such as the immune response or platelet activation. However, additional investigations are required to better and more completely understand the mechanisms of EVs that are involved in brain inflammatory processes.
Neurodegenerative Diseases
Brain diseases can occur in multiple forms: infections (meningitis, encephalitis) [65], seizures (epilepsy) [66], trauma (concussion, traumatic brain injury (TBI)) [67], vascular conditions (stroke [68]), autoimmune conditions (vasculitis, multiple sclerosis (MS)) [69], neurodegenerative conditions (Parkinson’s disease (PD), Alzheimer’s disease (AD) [70], and tumors (glioblastoma, brain tumor) [71]. While some of these brain diseases have diagnosis predictors [71], others still face the lack of effective molecular or biological markers, for example, neurodegenerative diseases (NDs).
Given the role of neurons in trans-synaptic exchanges, it is not surprising that EVs are regarded to be a vector for the dissemination of pathological alterations in the brain. The propagation of well-described pathological proteins contained in EVs such as tau, amyloid-β (Aβ) peptide or α-synuclein has already been depicted [72]. The research conducted by Wang et al. highlighted the release and trans-synaptic transmission of Tau protein by EVs of cultured cortical neurons [73]. EVs were able to mediate the neuron-to-neuron transportation of tau protein via direct transmission, which could contribute to the spreading of tau protein involved in Alzheimer’s disease and other tauopathies [73]. The pathogenesis of Alzheimer’s disease includes another identified hallmark, the Aβ peptide [74].
Astrocyte-derived EVs have also been reported to spread or exacerbate neuropathology. A quantitative proteomics study comparing brain-derived EVs from a nontransgenic (NTg) and a transgenic amyotrophic lateral sclerosis (ALS) animal model indicated that astrocyte- and neuron-derived EVs from ALS animal models carry a misfolded and aggregated pathogenic protein, SOD1 [75]. These findings propose that EVs containing misfolded and pathogenic proteins will be transmitted into recipient cells, thus contributing to the mechanism of disease propagation [75].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10092147
References
- Gassama, Y.; Favereaux, A. Emerging Roles of Extracellular Vesicles in the Central Nervous System: Physiology, Pathology, and Therapeutic Perspectives. Front. Cell. Neurosci. 2021, 15, 7.
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232.
- Brenna, S.; Krisp, C.; Altmeppen, H.C.; Magnus, T.; Puig, B. Brain-Derived Extracellular Vesicles in Health and Disease: A Methodological Perspective. Int. J. Mol. Sci. 2021, 22, 1365.
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359.
- Yue, B.; Yang, H.; Wang, J.; Ru, W.; Wu, J.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. Exosome Biogenesis, Secretion and Function of Exosomal MiRNAs in Skeletal Muscle Myogenesis. Cell Prolif. 2020, 53, e12857.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Pouso, M.R.; Cairrao, E. Effect of Retinoic Acid on the Neurovascular Unit: A Review. Brain Res. Bull. 2022, 184, 34–45.
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The Neurovascular Unit-Concept Review. Acta Physiol. 2014, 210, 790–798.
- Yu, X.; Ji, C.; Shao, A. Neurovascular Unit Dysfunction and Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 334.
- Hudson, N.; Campbell, M. Tight Junctions of the Neurovascular Unit. Front. Mol. Neurosci. 2021, 14.
- Huang, Z.; Wong, L.-W.; Su, Y.; Huang, X.; Wang, N.; Chen, H.; Yi, C. Blood-Brain Barrier Integrity in the Pathogenesis of Alzheimer’s Disease. Front. Neuroendocrinol. 2020, 59, 100857.
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes Regulate the Blood–Brain Barrier. Nature 2010, 468, 557–561.
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the Neurovascular Unit: Key Functions and Signaling Pathways. Nat. Neurosci. 2016, 19, 771–783.
- Tietz, S.; Engelhardt, B. Brain Barriers: Crosstalk between Complex Tight Junctions and Adherens Junctions. J. Cell Biol. 2015, 209, 493–506.
- Ahmed, T.A.; El-Badri, N. Pericytes: The Role of Multipotent Stem Cells in Vascular Maintenance and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1079, 69–86.
- Yao, Y.; Chen, Z.-L.; Norris, E.H.; Strickland, S. Astrocytic Laminin Regulates Pericyte Differentiation and Maintains Blood Brain Barrier Integrity. Nat. Commun. 2014, 5, 3413.
- Skoff, R.P. Gliogenesis in Rat Optic Nerve: Astrocytes Are Generated in a Single Wave before Oligodendrocytes. Dev. Biol. 1990, 139, 149–168.
- Thurgur, H.; Pinteaux, E. Microglia in the Neurovascular Unit: Blood–Brain Barrier–Microglia Interactions After Central Nervous System Disorders. Neuroscience 2019, 405, 55–67.
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424.
- Barateiro, A.; Brites, D.; Fernandes, A. Oligodendrocyte Development and Myelination in Neurodevelopment: Molecular Mechanisms in Health and Disease. Curr. Pharm. Des. 2016, 22, 656–679.
- Banerjee, S.; Bhat, M.A. Neuron-Glial Interactions in Blood-Brain Barrier Formation. Annu. Rev. Neurosci. 2007, 30, 235–258.
- Segarra, M.; Aburto, M.R.; Hefendehl, J.; Acker-Palmer, A. Neurovascular Interactions in the Nervous System. Annu. Rev. Cell Dev. Biol. 2019, 35, 615–635.
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2017, 23, 6206–6214.
- Marostica, G.; Gelibter, S.; Gironi, M.; Nigro, A.; Furlan, R. Extracellular Vesicles in Neuroinflammation. Front. Cell Dev. Biol. 2021, 8, 1841.
- Busatto, S.; Morad, G.; Guo, P.; Moses, M.A. The Role of Extracellular Vesicles in the Physiological and Pathological Regulation of the Blood-Brain Barrier. FASEB Bioadv. 2021, 3, 665–675.
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851.
- Stremersch, S.; De Smedt, S.C.; Raemdonck, K. Therapeutic and Diagnostic Applications of Extracellular Vesicles. J. Control. Release 2016, 244, 167–183.
- Pinheiro, A.; Silva, A.M.; Teixeira, J.H.; Gonçalves, R.M.; Almeida, M.I.; Barbosa, M.A.; Santos, S.G. Extracellular Vesicles: Intelligent Delivery Strategies for Therapeutic Applications. J. Control. Release 2018, 289, 56–69.
- Oscar, P.; Wiklander, B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; Andaloussi, S.E.L. Advances in Therapeutic Applications of Extracellular Vesicles. Sci. Transl. Med. 2019, 11, eaav8521.
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What Is a Pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451–455.
- Yuan, X.; Wu, Q.; Wang, P.; Jing, Y.; Yao, H.; Tang, Y.; Li, Z.; Zhang, H.; Xiu, R. Exosomes Derived From Pericytes Improve Microcirculation and Protect Blood–Spinal Cord Barrier After Spinal Cord Injury in Mice. Front. Neurosci. 2019, 13.
- Willis, C.M.; Nicaise, A.M.; Bongarzone, E.R.; Givogri, M.; Reiter, C.R.; Heintz, O.; Jellison, E.R.; Sutter, P.A.; TeHennepe, G.; Ananda, G.; et al. Astrocyte Support for Oligodendrocyte Differentiation Can Be Conveyed via Extracellular Vesicles but Diminishes with Age. Sci. Rep. 2020, 10, 828.
- Steiner, J.P.; Gardner, K.; Baines, A.; Bennett, V. Synapsin I: A Regulated Synaptic Vesicle Organizing Protein. Brain Res. Bull. 1987, 18, 777–785.
- Wang, S.; Cesca, F.; Loers, G.; Schweizer, M.; Buck, F.; Benfenati, F.; Schachner, M.; Kleene, R. Synapsin I Is an Oligomannose-Carrying Glycoprotein, Acts as an Oligomannose-Binding Lectin, and Promotes Neurite Outgrowth and Neuronal Survival When Released via Glia-Derived Exosomes. J. Neurosci. 2011, 31, 7275–7290.
- Bélanger, M.; Magistretti, P.J. The Role of Astroglia in Neuroprotection. Dialogues Clin. Neurosci. 2009, 11, 281–295.
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The Role of Astrocytes in Oxidative Stress of Central Nervous System: A Mixed Blessing. Cell Prolif. 2020, 53, e12781.
- Ganfornina, M.D.; Do Carmo, S.; Lora, J.M.; Torres-Schumann, S.; Vogel, M.; Allhorn, M.; González, C.; Bastiani, M.J.; Rassart, E.; Sanchez, D. Apolipoprotein D Is Involved in the Mechanisms Regulating Protection from Oxidative Stress. Aging Cell 2008, 7, 506–515.
- Ganfornina, M.D.; Do Carmo, S.; Martínez, E.; Tolivia, J.; Navarro, A.; Rassart, E.; Sanchez, D. ApoD, a Glia-Derived Apolipoprotein, Is Required for Peripheral Nerve Functional Integrity and a Timely Response to Injury. Glia 2010, 58, 1320–1334.
- Pascua-Maestro, R.; González, E.; Lillo, C.; Ganfornina, M.D.; Falcón-Pérez, J.M.; Sanchez, D. Extracellular Vesicles Secreted by Astroglial Cells Transport Apolipoprotein D to Neurons and Mediate Neuronal Survival Upon Oxidative Stress. Front. Cell. Neurosci. 2018, 12, 526.
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil Is in the Details. J. Neurochem. 2016, 139, 136–153.
- Bazan, N.G.; Halabi, A.; Ertel, M.; Petasis, N.A. Chapter 34—Neuroinflammation. In Basic Neurochemistry, 8th ed.; Brady, S.T., Siegel, G.J., Albers, R.W., Price, D.L., Eds.; Academic Press: New York, NY, USA, 2012; pp. 610–620. ISBN 978-0-12-374947-5.
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and Foe for Ischemic Stroke. J. Neuroinflammation 2019, 16, 142.
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation Pathways: A General Review. Int. J. Neurosci. 2017, 127, 624–633.
- Naveed, M.; Zhou, Q.-G.; Han, F. Cerebrovascular Inflammation: A Critical Trigger for Neurovascular Injury? Neurochem. Int. 2019, 126, 165–177.
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089.
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670.
- Babu, R.; Bagley, J.H.; Di, C.; Friedman, A.H.; Adamson, C. Thrombin and Hemin as Central Factors in the Mechanisms of Intracerebral Hemorrhage–Induced Secondary Brain Injury and as Potential Targets for Intervention. Neurosurg. Focus 2012, 32, E8.
- Shao, Z.; Tu, S.; Shao, A. Pathophysiological Mechanisms and Potential Therapeutic Targets in Intracerebral Hemorrhage. Front. Pharmacol. 2019, 10, 1079.
- Yates, A.G.; Anthony, D.C.; Ruitenberg, M.J.; Couch, Y. Systemic Immune Response to Traumatic CNS Injuries—Are Extracellular Vesicles the Missing Link? Front. Immunol. 2019, 10, 2723.
- Vaz, A.R.; Pinto, S.; Ezequiel, C.; Cunha, C.; Carvalho, L.A.; Moreira, R.; Brites, D. Phenotypic Effects of Wild-Type and Mutant SOD1 Expression in N9 Murine Microglia at Steady State, Inflammatory and Immunomodulatory Conditions. Front. Cell. Neurosci. 2019, 13, 109.
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-Derived Microparticles Mediate Neuroinflammation after Traumatic Brain Injury. J. Neuroinflammation 2017, 14, 47.
- Bhargava, P.; Nogueras-Ortiz, C.; Chawla, S.; Bæk, R.; Jørgensen, M.M.; Kapogiannis, D. Altered Levels of Toll-Like Receptors in Circulating Extracellular Vesicles in Multiple Sclerosis. Cells 2019, 8, 1058.
- Ibáñez, F.; Montesinos, J.; Ureña-Peralta, J.R.; Guerri, C.; Pascual, M. TLR4 Participates in the Transmission of Ethanol-Induced Neuroinflammation via Astrocyte-Derived Extracellular Vesicles. J Neuroinflammation 2019, 16, 136.
- Peng, N.; Su, L. Progresses in Understanding Trauma-Induced Coagulopathy and the Underlying Mechanism. Chin. J. Traumatol. 2017, 20, 133–136.
- Levi, M.; van der Poll, T. Two-Way Interactions between Inflammation and Coagulation. Trends Cardiovasc. Med. 2005, 15, 254–259.
- Biró, É.; Sturk-Maquelin, K.N.; Vogel, G.M.T.; Meuleman, D.G.; Smit, M.J.; Hack, C.E.; Sturk, A.; Nieuwland, R. Human Cell-Derived Microparticles Promote Thrombus Formation in Vivo in a Tissue Factor-Dependent Manner. J. Thromb. Haemost. 2003, 1, 2561–2568.
- Morel, N.; Morel, O.; Petit, L.; Hugel, B.; Cochard, J.-F.; Freyssinet, J.-M.; Sztark, F.; Dabadie, P. Generation of Procoagulant Microparticles in Cerebrospinal Fluid and Peripheral Blood After Traumatic Brain Injury. J. Trauma Acute Care Surg. 2008, 64, 698–704.
- Nekludov, M.; Mobarrez, F.; Gryth, D.; Bellander, B.-M.; Wallen, H. Formation of Microparticles in the Injured Brain of Patients with Severe Isolated Traumatic Brain Injury. J. Neurotrauma 2014, 31, 1927–1933.
- Nieuwland, R.; Berckmans, R.J.; Rotteveel-Eijkman, R.C.; Maquelin, K.N.; Roozendaal, K.J.; Jansen, P.G.; ten Have, K.; Eijsman, L.; Hack, C.E.; Sturk, A. Cell-Derived Microparticles Generated in Patients during Cardiopulmonary Bypass Are Highly Procoagulant. Circulation 1997, 96, 3534–3541.
- Lalic-Cosic, S.; Dopsaj, V.; Kovac, M.; Mandic-Markovic, V.; Mikovic, Z.; Mobarrez, F.; Antovic, A. Phosphatidylserine Exposing Extracellular Vesicles in Pre-Eclamptic Patients. Front. Med. 2021, 8, 761453.
- Owens, A.P.; Mackman, N. Microparticles in Hemostasis and Thrombosis. Circ. Res. 2011, 108, 1284–1297.
- Berckmans, R.J.; Sturk, A.; van Tienen, L.M.; Schaap, M.C.L.; Nieuwland, R. Cell-Derived Vesicles Exposing Coagulant Tissue Factor in Saliva. Blood 2011, 117, 3172–3180.
- Spronk, H.M.H.; ten Cate, H.; van der Meijden, P.E.J. Differential Roles of Tissue Factor and Phosphatidylserine in Activation of Coagulation. Thromb. Res. 2014, 133, S54–S56.
- Poplin, V.; Boulware, D.R.; Bahr, N.C. Methods for Rapid Diagnosis of Meningitis Etiology in Adults. Biomark. Med. 2020, 14, 459–479.
- Scharfman, H.E. The Neurobiology of Epilepsy. Curr. Neurol. Neurosci. Rep. 2007, 7, 348–354.
- Mckee, A.C.; Daneshvar, D.H. Chapter 4—The Neuropathology of Traumatic Brain Injury. In Handbook of Clinical Neurology; Grafman, J., Salazar, A.M., Eds.; Traumatic Brain Injury, Part I; Elsevier: Amsterdam, The Netherlands, 2015; Volume 127, pp. 45–66.
- Wolfe, C.D.A. The Impact of Stroke. Br. Med. Bull. 2000, 56, 275–286.
- Kornek, B.; Lassmann, H. Neuropathology of Multiple Sclerosis—New Concepts. Brain Res. Bull. 2003, 61, 321–326.
- Gammon, K. Neurodegenerative Disease: Brain Windfall. Nature 2014, 515, 299–300.
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251.
- Chivet, M.; Hemming, F.; Pernet-Gallay, K.; Fraboulet, S.; Sadoul, R. Emerging Role of Neuronal Exosomes in the Central Nervous System. Front. Physiol. 2012, 3, 145.
- Wang, Y.; Balaji, V.; Kaniyappan, S.; Krüger, L.; Irsen, S.; Tepper, K.; Chandupatla, R.; Maetzler, W.; Schneider, A.; Mandelkow, E.; et al. The Release and Trans-Synaptic Transmission of Tau via Exosomes. Mol. Neurodegener. 2017, 12, 5.
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the Amyloid-Beta Peptide. J. Alzheimers Dis. 2010, 19, 311–323.
- Silverman, J.M.; Christy, D.; Shyu, C.C.; Moon, K.-M.; Fernando, S.; Gidden, Z.; Cowan, C.M.; Ban, Y.; Stacey, R.G.; Grad, L.I.; et al. CNS-Derived Extracellular Vesicles from Superoxide Dismutase 1 (SOD1)G93A ALS Mice Originate from Astrocytes and Neurons and Carry Misfolded SOD1. J. Biol. Chem. 2019, 294, 3744–3759.
- Li, N.; Wu, Y.; Zhu, L.; Huang, Y.; Liu, Z.; Shi, M.; Soltys, D.; Zhang, J.; Chang, Q. Extracellular Microvesicles-Derived from Microglia Treated with Unaggregated α-Synuclein Attenuate Mitochondrial Fission and Toxicity-Induced by Parkinsonian Toxin MPP+. Biochem. Biophys. Res. Commun. 2019, 517, 642–647.
- Yuyama, K.; Igarashi, Y. Exosomes as Carriers of Alzheimer’s Amyloid-ß. Front. Neurosci. 2017, 11, 229.
- Yuyama, K.; Sun, H.; Sakai, S.; Mitsutake, S.; Okada, M.; Tahara, H.; Furukawa, J.; Fujitani, N.; Shinohara, Y.; Igarashi, Y. Decreased Amyloid-β Pathologies by Intracerebral Loading of Glycosphingolipid-Enriched Exosomes in Alzheimer Model Mice. J. Biol. Chem. 2014, 289, 24488–24498.
