Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Lipid based nanoparticles effectively transport delicate molecules for therapeutic purposes, protecting them from degradation, increasing their stability in the blood circulation and allowing to convey and release the transported substances in specific areas of the body. The formulation of liposomes, cubosomes and hexosomes can be tuned to obtain pH- or temperature responsive nanoparticles. Understanding the response to such external stimuli is of paramount importance for the design and production of efficient drug delivery systems.
- lipid nanoparticles
- drug delivery
- SAXS
- structural analysis
1. Introduction
Liposomes, cubosomes and hexosomes allow for incorporating lipophilic and hydrophilic drugs: their formulations can be stabilized with additives that increase the embedded drug stability and offer the possibility to trigger the release of the substances, making them ideal vectors for target drug delivery systems [50,51].
Adjusting the nanoparticle composition allows for creating drug delivery tools with specific responsive characteristics that can be investigated in situ with SAXS. With this information, lipid nanoparticles for therapeutic use can be designed according to the administration route, or to the target site.
It was recently demonstrated in an experiment in vivo that cubosomes obtained by coupling selachil alcohol with Tween® 80 as a stabilizer were more effective in delivering the poorly water soluble drug Phenytoin to the brain, with respect to hexosomes derived from the same lipid, but using Pluronic® F127 as stabilizing agent [52]. This better performance was attributed to the increased negative curvature of the lipid bilayer induced by Phenytoin in the presence of Tween80, which is more internalized in the lipid bilayer with respect to Pluronic F127. Usually, the lipid composition must be adjusted in order to change the lipid curvature to induce a phase transition. In the reported study, simple formulations composed of one single lipid were produced, and the phase-change was obtained by tuning the stabilizer.
Understanding the dissolution mechanism is a critical aspect of delivery systems design: in this respect, SAXS offers the possibility to study in situ the lipolysis during digestion. Simultaneous Small and Wide-Angle X-ray Scattering (SWAXS) allows for thoroughly evaluating the extent of drug dissolution, as the changes in crystalline structure of the internalized drugs can also be observed [54]. The formation of novel structures during lipolysis—thus, during the digestion of the orally administered formulation—can affect the solubility of the nanoparticles, reducing the drug absorption. As recently reported, drug carriers based on natural surfactants have great potential for the production of Self Emulsifying Drug Delivery Systems (SEDDS). It was recently demonstrated in a study on coupled in situ SAXS and ex situ Cryo-TEM, that during in vitro lipolysis, SEDDS undergo a structural evolution that is correlated with the amount of the surfactant in the formulation [39].
Elucidating the fate of nanostructured lipid nanoparticles when they come into contact with target cells is of paramount importance. Recently, the phase-change of non-lamellar lipid nanoparticles when they interact with cells was studied in situ with SAXS. A 3D model simulating the endothelium of human umbilical vein (HUVE) was developed, and the evolution of cubosomes to hexosomes during circulation was investigated [41]. Umbilical cells were seeded on the inner surface of a glass capillary, and the cubosomes’ dispersion was subsequently flown through it (Figure 1). The flow-through cell was exposed to X-rays, and the phase transition of cubosomes into hexosomes during contact with the cells was observed. These experiments demonstrated the potentiality of a structural study in real time.
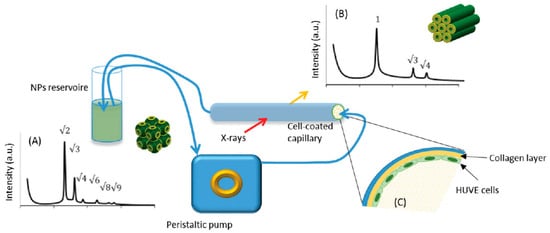
Figure 1. Scheme of the experimental setup. Phase transition from (A) cubosomes to (B) hexosomes, (the respective Bragg peaks indexing are indicated), when in contact with a glass tube coated with human umbilical vein endothelium cells (HUVE), was observed in situ with SAXS. (C) Detail of the observation cell coated with human umbilical vein endotheliumcells. (Adapted from [41]).
2. External Stimuli Responsive Lipid Nanoparticles
To design drug delivery nanoparticles that are responsive to external stimuli, understanding how to tune the phase transition is a critical aspect. It is usually induced by a change in temperature or in the pH of the medium, and it can be exploited to determine the drug-release extent.
By loading lipid nanoparticles composed of MO and oleic acid (OA) with the antitumor drug Doxorubicin and with a natural oil obtained from Brucea javanica (BJO)—a traditional medical herb with antiproliferative properties on cancer cells—Li and collaborators obtained pH-sensitive lipid nanoparticles capable of converting from hexagonal to cubic crystalline phase to emulsified microemulsions, in response to the lower pH in proximity and inside tumor cells. SAXS analysis demonstrated the pH responsiveness and the effect of Brucea Javanica oil on the nanoparticles’ stability (Figure 2). At pH 7.4, hexosomes with an internal HII-phase structure were formed: at a lower pH (6.8), the crystalline structure changed to cubosomes, with an inner structure where two phases (Pn3m and Im3m) coexist [34].
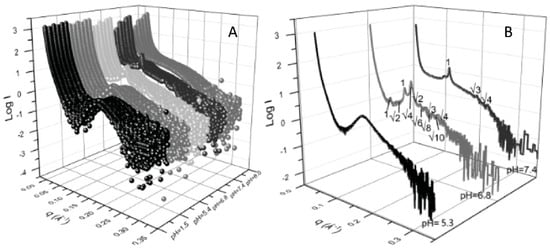
Figure 2. (A) SAXS patterns obtained from nanoparticles of different composition (front to back: MO:OA, MO:0.9OA:0.1BJO, MO:0.8OA:0.2BJO, MO:0.7OA:0.3BJO, MO:0.6OA:0.4BJO and MO:0.5OA:0.5BJO) collected at different pH (pH = 1.5, 5.3, 6.8, 7.4, and 8.0, front to back); (B) SAXS pattern of lipid nanoparticles loaded with oleic acid and Brucea javanica oil (MO:0.9OA:0.1BJO) collected at pH 7.4 (healthy cells), 6.8 (tumor cells proximity) and 5.4 (tumor cells endosomes). The Bragg peaks indexing of inverted hexagonal (pH 7.4) and inverted cubic (pH 6.8) liquid crystalline phases is indicated (Reproduced with permission from [34]).
By modifying their composition, it is possible to tailor the response to the external conditions and to create a “library” of pH- and temperature-responsive nanoparticles, whose crystalline structure can be tuned for fast (cubic) or slow (hexagonal) drug release. Recently, lyotropic liquid crystals with cubic Fd3M symmetry obtained from MO were doped with various types and amounts of fatty acids (FA) and fatty acetates (FAc) with different chain lengths (Figure 3). By adjusting the nanoparticles composition, it was possible to produce a collection of hybrid MO-FA–FAc micellar nanoparticles which exhibited a Fd3m structure. As demonstrated by in situ SAXS, these cubosomes underwent cubic to lamellar and cubic to hexagonal phase transitions in response to temperature and pH changes, respectively, depending on the chain length, the amount and the respective molar ratio of FA and FAc incorporated in the formulation [35].
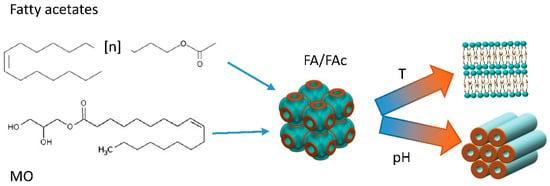
Figure 3. Cartoon representing the experiment reported in [35]. The phase transition from cubosomes to hexosomes of MO nanoparticles produced with different amounts of Fac and FA in different molar ratios can be controlled with temperature and pH variations.
In another experiment, a set of synthetic ionizable aminolipids was designed and used to dope MO nanoparticles stabilized with the surfactant Pluronic® F-127. SAXS allowed for investigating the phase transition of MO in response to different pH of the several formulations obtained by adjusting the nanoparticles’ composition [36].
3. Hybrid Lipid-Based Nanoparticles
Lipids can be associated with other biocompatible molecules, such as polysaccharides, in order to induce the formation of structured mesophases with high stability and specificity for applications where bioadhesion is desired. Coupling MO with Pluronic® F127 as a stabilizer, chitosan-N-arginine and alginate lead to the formation of pH responsive cubosome nanoparticles. SAXS experiments allowed for determining the proper amount of stabilizer needed to obtain nanoparticles characterized by cubic symmetry, to gain insight in the phase transition in response to pH change as well as on the distribution of chitosan-N-arginine inside the lipid structure [37].
Lipids can be also coupled with inorganic materials, with a great potential for localized drug release. Caselli and collaborators studied the behavior of hybrid paramagnetic nanoparticles when exposed to a magnetic field: they exploited the capability of lipidic-ordered liquid crystals to change the phase from cubic to hexagonal, to trigger the localized drug release. The phase-change was accompanied by a massive water ejection that could promote the release of hydrophilic drugs embedded in the lipid nanoparticles. By coupling superparamagnetic iron oxide nanoparticles with 1-monoolein (GMO) nanoparticles, they produced hybrid cubosomes with Pn3m cubic symmetry, which changed to hexagonal in response to the heat generated by the magnetic nanoparticles exposed to a low-frequency alternating magnetic field [42]. With SAXS, it was possible to characterize in situ the lipid phase transition either during the exposure to temperature only, or to an alternating magnetic field. In the absence of a magnetic field, the phase transition from Pn3m to HII was observed at temperatures compatible with physiological conditions (below 40 °C) [55]. Both pure GMO cubosomes and hybrid nanoparticles were exposed to the alternating magnetic field: SAXS data confirmed the thermotropic effect of the superparamagnetic iron oxide on the crystalline structure of the hybrid cubosomes once exposed to the magnetic field, while no phase-change was observed in the pure cubosomes. SAXS data analysis also revealed a pearl-necklace-like arrangement of the hybrid magnetic cubosomes after 210 s of exposure to the magnetic field (Figure 4).
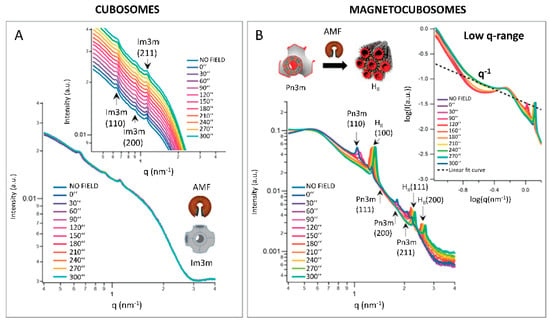
Figure 4. Response of (A) GMO cubosomes and (B) superparamagnetic cubosomes (showing a phase transition from Pn3m (cubic) to HII (hexagonal) crystalline phase) exposed to an alternated magnetic field. The Miller Indexes for the respective crystalline phases are indicated. Each SAXS profile corresponds to a different application time of the magnetic field. Inset in (A) highlights the scattering peaks of cubosomes. Inset in (B) represents a magnification of the low-q region of the scattering profile. The pearl-necklace-like arrangement of magnetocubosomes after 210 s of exposure to the magnetic field is evidenced by the appearance of a q−1 scalar law. (Reproduced with permission from [42]).
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14122704
This entry is offline, you can click here to edit this entry!
