Arsenic (As), a non-metallic element located in group VA and period IV of the periodic table, is widely found in nature. Its average concentration in the Earth’s crust is approximately 2~5 mg/kg, which ranks as the 20th position of the elements forming the Earth’s crust. Trace amounts of arsenic can exist in soil, water, minerals, plants and normal human tissues. Arsenic presents in the forms of inorganic arsenic and organic arsenic, specifically As3+ and As5+. Arsenic is a toxic and carcinogenic element. Inorganic arsenic is more toxic than organic arsenic, and As3+ is around 60 times more toxic than As5+. Inorganic arsenic species mainly exist in the form of arsenate in water, such as H3AsO4, H3AsO3, H2AsO4−, H2AsO3−, AsO33− and AsO43−.
1. Small Organic Molecules
In recent years, some fluorescent probes based on organic small molecules have been reported
[1][2]. In this regard, the fluorescence detection technique based on organic small molecules has achieved an unprecedented success, not only because of its low detection limit, but also due to its low cost, easy operability and high selectivity
[3]. In fact, fluorescence probes are widely used to detect biologically relevant analytes.
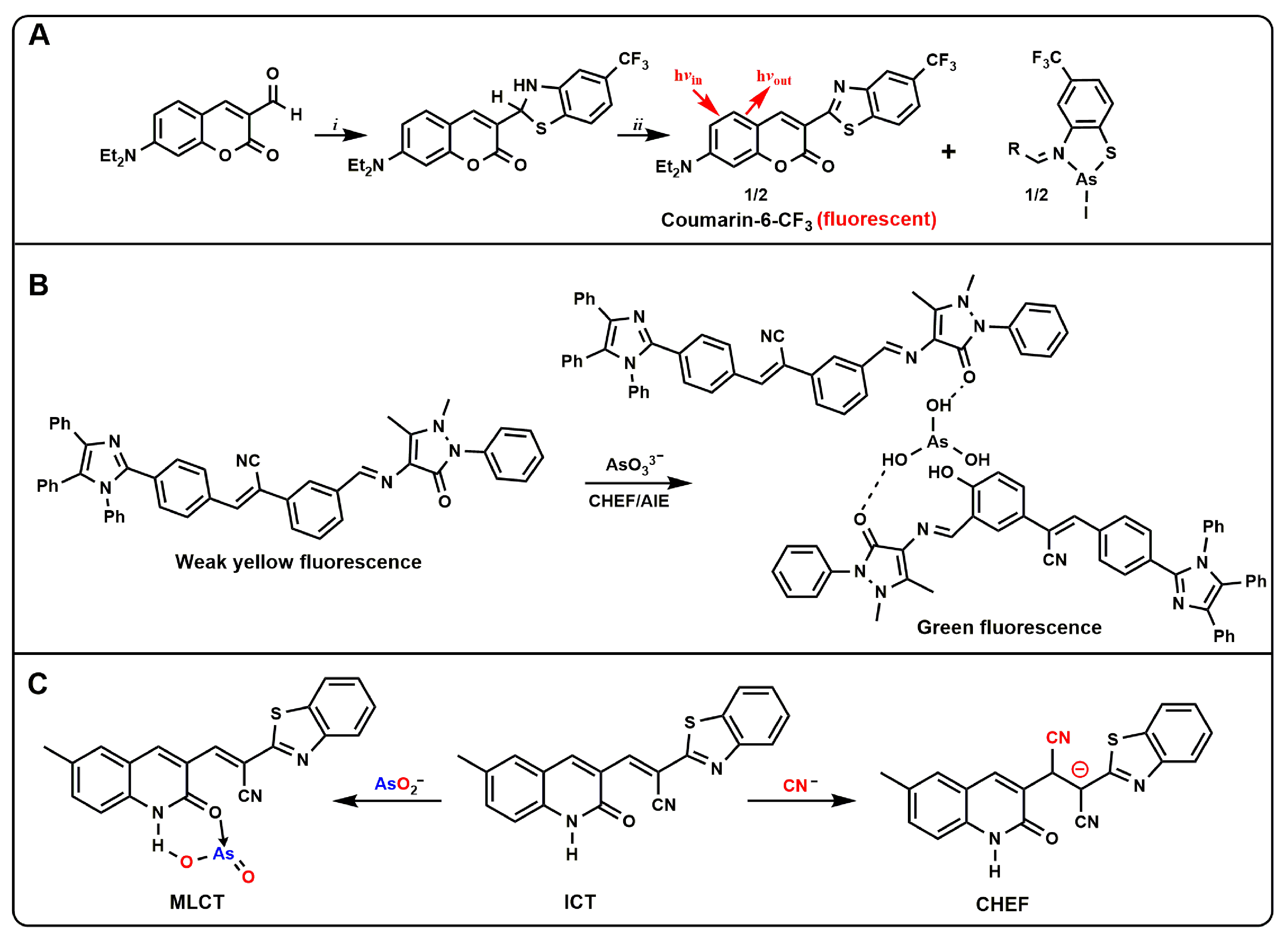
Harrop et al. developed a small-molecule probe ArsenoFluor1 (AF1,
Figure 1A)
[4]. The AF1 probe could be combined with As
3+ and produce a highly fluorescent benzothiazole molecule, a common dye called coumarin-6. The limit of detection (LOD) was estimated to be 0.53 nM (0.24 ppb), suggesting that AF1 might be used to monitor As
3+ levels well below the EPA standard of 10 ppb. Based on this structure, the questionable set also replaced -CF
3 with -H, which could also be applied to the detection of As
3+, and expanded the database of AF1 probes
[5]. Song et al. reported a new tetraphenylimidazol-based probe (TBAB,
Figure 1B) functionalized with Schiff base for the detection of arsenic ions in water
[6]. After the addition of arsenic ions, the chelation of TBAB with arsenic activated the AIE properties, resulting in enhanced fluorescence and a distinct fluorescence change from pale yellow to green that was visible to the naked eye. The probe could selectively detect arsenic in the presence of interfering substances with an LOD less than 0.7 ppb, which was well below the limit set by the WHO. A pyrene-based oxacalix [4]arene-Ce
3+ complex (L–Ce) based on light-induced electron transfer (PET) and chelation-enhanced fluorescence (CHEF) was developed
[7]. The complex could selectively detect As
5+ and Cr
6+ through a fluorescence-quenching response simultaneously. This L–Ce “turn-off” fluorescent probe for the detection of AsO
43− and CrO
42− could achieve an LOD of 2 ppb and 93 ppb, respectively. Similarly, Kumar et al. reported a quinoline acrylonitrile probe based on metal–ligand charge transfer (MLCT) and CHEF (
Figure 1C)
[8]. The probe was prepared from 6-methyl-2-oxo-1,2-dihydroquinoline-3-carbaldehyde with benzothiazole-2-acetonitrile. The probe showed a selective “off–on” fluorescence response to AsO
2− and CN
−, and could simultaneously detect arsenate by colorimetric visualization. The probe could bind to AsO
2− and CN
− in a 1:1 ratio and was undisturbed by other competing ions in the pH ranges 2–10 and 3–8, respectively. The LOD of AsO
2− by using spectrophotometry and RGB color tool were measured to be 24 ppb and 498 ppb, respectively, while CN
− detection by spectrofluorimetry detected down to 1 ppb. The probe was successfully applied to the detection of cyanide and arsenite in tap water and well water.
Figure 1. (A) Synthesis of AF1 and proposed As3+ response; (B) Schematic of As3+ detection using the probe TBAB; (C) The Proposed mechanism of chromogenic and fluorescence enhancement of the probe in the presence of AsO2− and CN−.
2,4-dinitrophenyl hydrazones are of great interest because they can interact with analytes via forming strong hydrogen bonds. Padmini et al. developed a visual probe based on 2,4-dinitrophenyl hydrazine framework for the detection of highly toxic As
3+ in water
[9]. Probe PHTH (E)-4- [{2-(2,4–dinitrophenyl) hydrazono}benzene 1,3-diol] was synthesized by 2,4-dinitrophenyl hydrazine, 2,4-dihydroxy benzaldehyde and ethanol. Experimental studies clearly demonstrated the high selectivity of the probe for As
3+ compared with other competing metal ions. Furthermore, the addition of As
3+ to the probe caused the PHTH solution to change from orange to purple under UV light in DMSO solvent. The LOD of the aqueous medium was calculated to be 0.35 μM.
Arsenic detection has not only been widely used in ambient water samples, but also in the development of intracellular detection. Annaraj et al. reported a fluorescent probe for the simultaneous detection of AsO
2− and H
2PO
4− in zebrafish embryos
[10]. The probe was based on the red fluorescent zinc complex (QAZn) and had an extremely strong direct binding to AsO
2− and H
2PO
4−. In the presence of AsO
2−, it was firstly bound to the QAZn and triggered the intramolecular charge transfer (ICT) process, which led to a blue shift of the emission peak. After that, the addition of H
2PO
4− caused the dissociation of Zn(II) from QAZn-AsO
2 complex and the released free ligand restored green fluorescence. Meanwhile, it has been further exploited for live cell studies in zebrafish embryos. Rhodamine, as a kind of dye with excellent photochemical properties, is often used as a fluorescent signal molecule. Banerjee et al. designed a molecular probe rich in rhodamine, PBCMERI-23 (3′,6′-bis-(ethylamino)-2-((2-hydroxy-5-methylbenzylidene)amino)-2′,7′-dimethylspiro [isoindoline-1,9′-xanthen]-3-one)
[11]. The simple, immediate and cost-effective luminescence probe enabled As
3+ to be selectively detected in aqueous media at an LOD level of 0.164 ppb. Meanwhile, the level of As
3+ was monitored in different cells, and the probe showed flashing yellow fluorescence, which indicated that the probe had good cell permeability and biological applicability. Recently, this group has synthesized a naphthalene additive luminophore
N-((4-((naphthalene-5-ylimino) methyl) phenyl) methylene) naphthalene-1-amine (NPN) with various properties. Based on “arsenoselective azomethine hydrolysis” (ASAH), NPN exhibited excellent fluorescence performance when interacting with As
3+. NPN could monitor As
3+ in different natural water sources in real time by a “turn on” fluorescence response. Meanwhile, cells treated with As
3+ emitted a flashing blue emission fluorescence. The rapid fluorescence enhancement capability of the probe enhanced its potential for field applications.
2. Organic Frameworks
Metal organic frameworks (MOFs) are a class of crystalline porous material with a periodic network structure. They are composed of inorganic metal centers (metal ions or metal clusters) and bridged organic ligands connected to each other through self-assembly. Since the emergence, MOFs have shown great potential in a wide range of applications
[12]. In fact, MOFs exhibit excellent luminescence properties due to their π-rich bridging organic ligands as well as metallic nodes/clusters and adsorbed or functionalized luminescent guest molecules. In recent years, luminescent MOFs (L-MOFs) have exhibited potential in a variety of applications including environmental problems
[13][14][15][16].
Ghosh et al. synthesized a new hydrolytically stable luminescent Zn
2+ based cationic MOFs (iMOF-4C)
[17]. iMOF-4C was constructed from a tripodal neutral N-donor linker 1,1′-(5′-(4-(1
H-imidazol-1-yl) phenyl)-[1,1′:3′,1″-terphenyl]-4,4″-diyl)bis(1
H-imidazole) and a d
10 metal ion for the simultaneous detection and removal of environmentally toxic oxygen anions (CrO
42−, Cr
2O
72−, HAsO
42−, and HAsO
32−) in aqueous media. The MOF based sensing probe showed accurate and sensitive recognition of these anions through its unique “on–off” fluorescent signal output and extremely fast response time. Xu et al. developed a ratiometric fluorescent biosensor based on acid phosphatase and hemin loaded multifunctional Zn-based MOF (ACP/hemin@Zn-MOF) for As
5+ sensing
[18]. The intrinsic fluorescence (452 nm) in ACP/hemin@Zn-MOF was derived from 2-aminoterephthalic acid ligand, and the hemin was characterized by peroxidase activity. ACP/hemin@Zn-MOF could catalyze the oxidation of o-phenylenediamine (OPD) to produce 2, 3-diaminophenazine (DAP) with a fluorescence signal at 564 nm and weaken the fluorescence intensity of ACP/hemin@Zn-MOF (452 nm). When present in the sample, ascorbic acid 2-phosphate (AAP) could be hydrolyzed by ACP to form ascorbic acid (AA), which hindered the oxidation of OPD and thus affected the fluorescence intensity at 564 nm. Furthermore, the addition of As
5+ could irreversibly poison ACP to prevent hydrolysis of AAP, resulting in the recovery of the fluorescence signal at 564 nm and the suppression of the signal at 452 nm again. The LOD of ACP/hemin@Zn-MOF for As
5+ was 1.05 μg/L. Based on the optimization of organic ligands, a novel Eu-MOF with the capability of AsO
43− emission sensing and trapping was constructed by the solvothermal method
[19]. The Eu-MOF nanostructures with dispersion characteristics clearly showed a “turn-on” fluorescence emission characteristic with obvious intensity contrast in the arsenate sample, which reduced the LOD to 17.8 nM for arsenate species in the aqueous environment. Subramanian et al. reported an MOF-derived magnetic porous carbon (MPC) composite
[20]. FAM labeled ssDNA was adsorbed on the surface of MPC composites and fixed by π-π stacking interaction, which resulted in the quenching of fluorescence intensity. When As(V) was added to the probe system, the strong binding of As(V) to MPC resulted in the spontaneous displacement of FAM-labeled ssDNA from the MPC material, and thus the fluorescence intensity was restored. Based on this principle, the fabricated sensor exhibited a highly sensitive fluorescence response to As
5+ in the range of 0–15 nM, with an LOD as low as 630 pM.
In recent years, covalent organic frameworks (COFs) have attracted much attention in fluorescence sensing due to their regular pore structure, stable π-conjugate frame, and easiness of synthesis
[21]. COFs are easy to functionalize and uses the specified group as the recognition sites in the framework. In particular, 2D COFs may be the best candidate for the detection of highly toxic ions such as As
3+. Yin and Liu reported functional COFs based on bipyridine (Dpy-TFPB) for the fluorescence “turn on” mode detection of As
3+ [22]. The nitrogen-based site of Dpy-TFPB was a highly selective receptor for As
3+, and its π bond acted as a signal responder. In the presence of As
3+, the combination with N group destroyed the photoinduced electron transfer (PET) process and exhibited obvious fluorescence. Dpy-TFPB showed high sensitivity and an ultra-low LOD of 8.86 nM was determined. To further improve the application of COFs in the detection of arsenic, Chen et al. used COFs as a fluorescence sensor for the first time to detect and adsorb organic arsenic in water
[23]. Two isoreticular crystalline and highly porous sp
2 carbon-conjugated COFs were synthesized, and were amidoxime-functionalized via post-synthetic modification (PSM). The long-range ordered and π-conjugated system ensured that two kinds of COFs were used as fluorescence sensors for the detection of representative organic arsenic roxasone (ROX) through a fluorescence-quenching response. The LOD of ROX for the two kinds of COFs were 6.5 nM and 12.3 nM, respectively.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27238497