Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Energy plants, industries, as well as other sources of carbon dioxide (CO2) result in global warming and affect the planet. CO2 is separated from flue gas during combustion using a variety of advanced separation techniques. There are several techniques involved, such as absorption, adsorption, chemical looping, membrane separation, and cryogenics.
- adsorption
- absorption
- chemical looping
- CO2 capture
1. Absorption
The ability of absorption to capture huge quantities of emissions from chemical factories and power plants has gained considerable attention in recent years. Chemical absorption is a reliable technique for CO2 separation in coal-fired power plants because it is well-suited for existing plants with high operating costs and limited infrastructure [1]. The chemical absorption of CO2 is a commercially viable technology due to its many advantages, including technical efficiency, handling capacity, and sophistication [2]. The potential absorbents and processes of absorption CO2 capture are highlighted in Table 1.
Table 1. Summary of absorption-based carbon capture.
| Type | Absorbent | Reactive Separator | Operating Conditions P, C, T, G | CO2 Capture (%), AC (kg/kg) | Kinetics/Mass Transfer | Ref. |
|---|---|---|---|---|---|---|
| Single solvent | MEA | Flow (SC) | C:8 −16; T:10–40; G:2–10 | 94, 0.4 | C2H4OHNH2(1)+CO2(g)+H2O↔C2H4OHNH+3(aq)+HCO−3(aq) | [3][4][5] |
| K2CO3 | Fixed-bed (Con-O, bench scale) | T:60 G:40 mL/min | 99.4, NA | NA | [6][7] | |
| Ammonia | Sieve plate (CC) | C:10–14; T:25–55 °C | 95–99, 1.2 | 2NH3(g)+CO2(g)⟺NH2COONH4(s) NH2COONH4(s)+H2O(g)⟺(NH4)2CO(s)CO3(s) |
[3][4] | |
| Piperazine | Stirred cell (SC, BS) | P:0.032 T:42 and 0.042 | 100, 0.32 | 1st order partial reaction occurs | [8] | |
| Ionic liquids | Double stirred cell (BS) | T:25–50; P:0.1; A:0.5–1.2 | 99.11 at 60 °C, | NA | [9][10][11][12] | |
| Mixed Solvents | DEA-K2CO3 | Split flow (CC, bench scale) | T:115 L:63.66 m3/h | 99, NA | Promoter selection is very critical. It is a reversible exothermic reaction CO2+K2CO3+H2O↔2KHCO3 |
[13] |
| PEI-SiO2 Alcohol/amine/water | Packed (bench scale) | L:33.66 m3/h | NA, NA | qsensible=CPΔmsolutionΔmCO2 | [14][15] | |
| BDA-DEEA | Packed (CC, BS) | T:40 (absorption) T:90 (desorption) G: 24.78 m3/h | 46 (HCL), 48 (HCC), 11(HCE) than MEA with 5 M | Carbamate and bicarbamate formations | [16] | |
| AMP-PZ | Packed (pilot) | L/G:2.9; packing height=10 m | 90, NA | - | [17][18][19][20][21][22][23][24] |
CO2 is separated from flue gas by absorption using a liquid sorbent [25][26]. It is possible to regenerate the sorbent via a regenerative process or stripping by depressurizing and/or heating. This is the latest and most advanced method for separating CO2 [27]. Potassium carbonate (K2CO3), monoethanolamine (MEA), and diethanolamine (DEA) are examples of common sorbents [28]. MEA is very reactive and absorbs more quickly, and it is quite inexpensive [27]. However, their main drawback is the substantial parasitic energy load in relation to solvent regeneration, which adversely affects the total effectiveness of systems combined with aqueous amine-based absorption processes [29]. DEA and other alkanolamines have also been employed for absorption, although they have comparable defects. Methyldiethanolamine (MDEA), a mixture of MEA and DEA, has been used with moderate success. It has higher CO2 loading capacity, and degradation and corrosion resistance, as well as cheaper regeneration costs, but lower rates of absorption [30][31][32][33][34][35].
Veawab et al. [36] reported that MEA is the most efficient aqueous alkanolamine for CO2 absorption, with a performance rate greater than 90%. Additionally, Aaron and Tsouris [37] reviewed various CO2 capture technologies and determined that MEA absorption is the most viable method for CO2 capture in CCS. Applying a solvent containing 30% MEA, a 1 t CO2/h absorption pilot plant was designed and experimentally validated in conjunction with a coal-fired power plant’s post-combustion capture technology [38]. In recent times, other adsorbents, including anion-functionalized ionic liquid and piperazine, have attracted a lot of attention [39]. Even though piperazine rapidly reacts compared to MEA, its use in CO2 absorption is more costly. Due to its higher volatility, it is still in the experimental phase [40]. The risk of amine degradation, which could lead to equipment corrosion, solvent loss, and the formation of volatile degradation compounds, is a significant barrier to the widespread adoption of this technology for the CCS [41][42], while environmental degradation has gone unnoticed.
Furthermore, amine emissions can deteriorate into nitramines and nitrosamines, which are highly toxic to human health and the environment. The chilled ammonia process captures CO2 using aqueous ammonium salts (including ammonium carbonate) and can regenerate the CO2 at elevated temperatures and pressures using waste heat, thereby minimizing the downstream compression [43]. There are fewer problems with this process than those caused by amine degradation.
Water’s use as a co-solvent, which has higher thermal characteristics than other co-solvents, is one of the key precursors for the high solvent regeneration energy of MEA [44]. In the context of CO2 absorption, the predicted regeneration energy for 30 wt. % aqueous MEA showed that more than 50% of the total energy was used to heat and vaporize the water co-solvent. The remaining energy was used to reverse the chemical interaction between CO2 and MEA at the same time [45][46]. Considering this, it was thought that either totally or partially substituting other organic diluents for water as co-solvents could potentially reduce solvent regeneration energy, since they effectively create water-free/water-lean hybrid solvents with poorer thermal properties than water [47][48][49][50][51][52][53]. Instead of vaporizing and heating the co-solvent, comparable to aqueous amines, the regeneration energy will be used more effectively to reverse acid gas chemisorption.
Additionally, hybrid water-free/water-lean solvents have been thoroughly studied in recent years, primarily for their CO2 capture applications [53][54][55]. They provide a wide range of potentially alluring substitutes to conventional aqueous amines [29][56]. The main objective of water-lean solvents is to preserve the chemical selectivity of water-based solvents, while enabling step gains in efficiency due to the lower specific heats of organics than water [29]. However, two problematic regions refute the claim of their attractiveness. The stated performance of these solvents when scaled up from lab-scale to industrial-scale settings has not been adequately examined due to a lack of availability of a few essential properties. This is predicted given the labor-intensive nature of experimental work, which makes it impossible to expand experimental testing to the broad range of transport and thermophysical parameters needed for precise and representative performance evaluation on an industrial scale. The second issue is that, when carried out on a lab scale, the potentiality of a particular solvent is typically demonstrated using a limited set of parameters, most notably the low enthalpy of absorption and high absorption capacity [57][58][59][60]. These two characteristics are indeed of great concern for chemical absorption procedures, but they are still unsuitable for accurately gauging the potential of the tested solvents for their intended use. However, they ignore significant trade-offs between competing environmental, economic, and operational factors. The results of a straightforward assessment can help direct the development of novel generating solvents [61][62].
However, these difficulties can be overcome if the proper tools or novel process configurations are available. Due to recent developments in computational power and thermodynamic modeling tools, the first issue can be resolved by scaling the data from lab to industrial operating conditions. The most appealing models for this application are molecular equations of state (EoSs) centered on the Statistical Associating Fluid Theory (SAFT) [63][64], due to their strong theoretical background, demonstrated correctness for a range of complex systems, and predictive abilities.
The solution to the second problem, which is to demonstrate the viability of a chosen solvent typically acknowledged using a limited number of requirements, may appear relatively apparent: add more evaluation criteria to the already-existing standard key performance indicators (KPIs). Moreover, making such a preference is more difficult because early design phases may not have access to information on a particular criterion [65]. There must be a justification for why certain criteria should be included or excluded when narrowing the search space among the numerous properties that are available [66][67][68]. The effect of solvent characteristics on economic metrics such as total capital expenditures (CAPEX) and operating expenditures (OPEX) typically serves as the foundation for justification. Through the careful process modeling of hypothetical solvents, Mota-Martinez et al. [69] ranked various solvent characteristics according to how they affected the process’ overall economics. Leclaire and Heldebrant [26] recently recommended the use of ideas from green engineering and chemistry to address problems with the advancement of CCUS technologies. They asserted that by applying the 12 + 12 principles of engineering and green chemistry [70], they could indirectly encourage the improvement of chemical processes’ economic attractiveness and efficiency, which goes beyond their environmental motivation. Similarly to this, it may be beneficial to consider sustainability, health, and safety issues while assessing the possibility of promising solvents for the removal of acid gas [71][72]. Figure 1 displays the schematic diagram for the absorption carbon capture process.
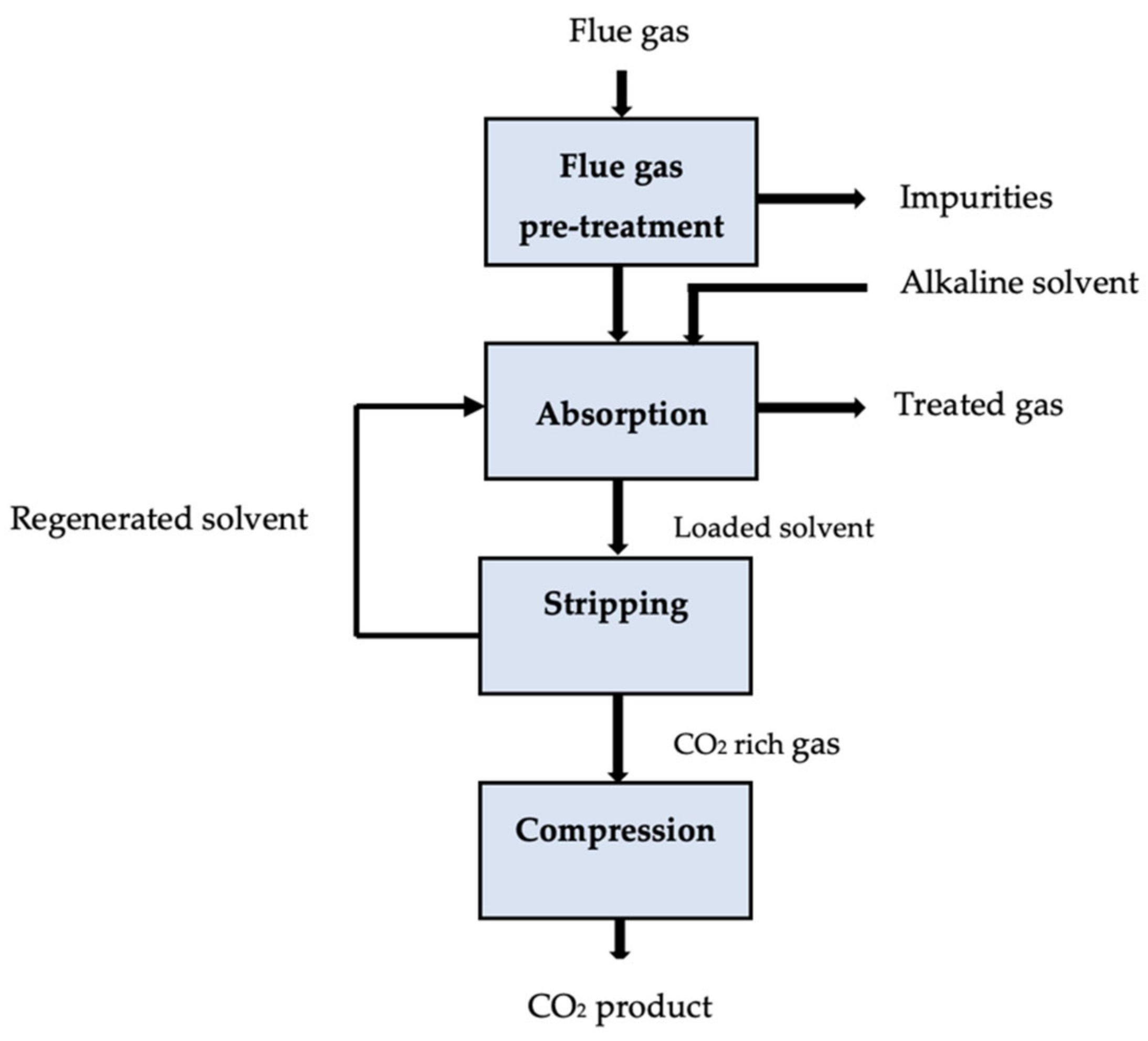
Figure 1. Schematic of an absorption carbon capture process [73].
2. Adsorption
The process of adsorption [26] involves molecules in liquids and gases adhering to solid surfaces by weak van der Waals interactions. Unlike liquid absorbent processes, solid adsorbents bind CO2 to their surfaces during adsorption. Selection criteria for this sorbent include a large surface area, high regeneration capability, and high selectivity. Common adsorbents include activated carbon, molecular sieves, zeolites, lithium zirconate, and hydrotalcite [74]. Table 2 highlights the potential adsorbents and adsorption parameters for CO2 capture.
It is possible to achieve CO2 adsorption by changing the pressure or temperature of a saturated sorbent. Pressure swing adsorption (PSA) is a commercially applied technology that recovers more than 85% of CO2 from power plants [75][76]. A solid adsorbent selectively adsorbs CO2 at high pressures, then the solid desorbs, releasing CO2 for low-pressure transport (usually atmospheric pressure). The temperature swing adsorption (TSA) releases the CO2 in the system by increasing its temperature through steam injection or hot air distribution [77]. A CO2 purity of over 95% and recovery of over 80% are possible when using CO2 regeneration, although regeneration is more time-consuming than PSA [78]. It was estimated that the operating costs of a particular TSA process ranged between USD 80 and 150 per tonne of CO2 captured [79]. Significant attention has been paid to developing CO2 capture sorbents from agricultural and industrial wastes to lower the overall cost of CO2 capture. An adsorption carbon capture process is shown in Figure 2.
Table 2. Summary of adsorption-based carbon capture.
| Adsorbent | Reactive Separator | Operating Conditions P, T, C, G | CO2 Capture (%), Ad-C (gCO2/gads) | Kinetics/Mass Transfer | Ref. |
|---|---|---|---|---|---|
| TEPA-Mg-MOF-74 | PBR (LS) | Regeneration temp is 250–300 °C | 4–4.9 wt. %, 8.31 mmol CO2/g absorbent, NA | N2 adsorption–desorption isotherm | [24] |
| ZX-APG, | PBR (3-bed, 8-step, VPSA, LS) | T:35; P: 0.007–0.008 | 85–95, NA, 73–82% CO2 purity | Langmuir adsorption isotherm is adopted | [80] |
| Activated carbon | PBR (1 bed, 3 step, VSA, LS) | Water vapour (H2O): 4.6 mol%, Vf: 44; TDes:100T: 60, ICC:11.2, Bd:0.493, Lg:50, P:0.113, PVP = 3, Trpt:3; SA:921.7, PV:0.37, Tads:35, | 69.5, NA | Dual-site Langmuir equation has been adopted | [81] |
| NPC10 | PBR (TSA, LS) | T: 25, P: 0.1, SA: 639 | NA, 0.041 | Langmuir adsorption isotherm | [82] |
| Fly ash + PEI + PEG | PBR (LS, TSA) | St: 24 h, P: 0.11, T: 70 | 4.5 at 85 °C | CO2+2RR'NH↔RR'NCOO−+RR'NH+2CO2+2H2O+RR'NCOO−↔2HCO−3+RR'NH+2 | [83] |
| ZX | MBA (LS, PSA) | Bed dimensions (m): FRR: 0.5; CT: 650; AT: 950; SA: 1873.9; 2b: 0.03, Nm: 36, W: 1.5; L:1.5; Xpth: 0.012 Bd: 0.65, Cs: 1.07, Dp: 3420, ε: 0.31 ks: 0.275 |
80, NA, 97% purity | Extended Langmuir isotherm was used | [84] |
| Rayon–HCM | PBR (TSA) | 97, 0.2 | Langmuir adsorption isotherm adopted | [85][86] |
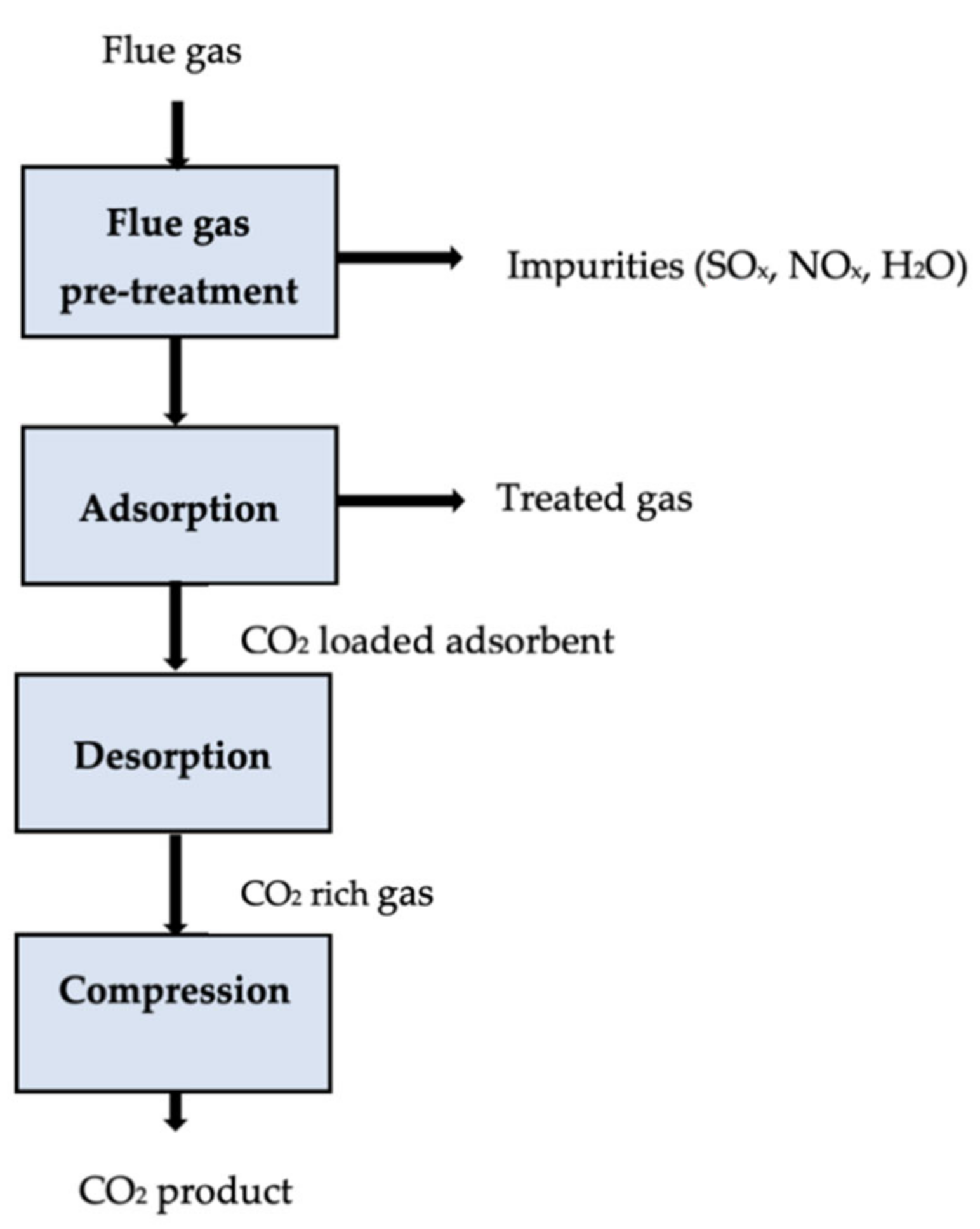
Figure 2. Schematic of an adsorption carbon capture process [73].
3. Chemical Looping Combustion
In contrast to oxy-fuel combustion, which uses pure oxygen for combustion, metal oxides are used as oxygen carriers in combustion. Metal oxides are reduced to metal during the process, while fuels are oxidized to create CO2 and water. In a subsequent stage, the metal is oxidized and recycled. The removal of water by condensation from the process byproducts is easy, but the separation of pure CO2 requires no energy. Numerous low-cost metal oxides, including Mn2O3, CuO, NiO, and Fe2O3, are suitable for this process. The potential sorbents and processes of chemical looping combustion are highlighted in Table 3.
Several researchers [87][88][89][90][91] have examined the performance efficiency of various metal oxides in this process. According to Adánez, de Diego [90], a metal oxide can be optimized by using support inert materials, but the selection of an inert material will vary depending on the characteristics of the metal oxide. Chemical looping combustion (CLC) was studied by Lyngfelt, Leckner [92] in a boiler consisting of two fluidized beds. Lyngfelt, Leckner [92] recently reviewed this technology. This process has been demonstrated to be a very promising CO2 capture technology by both Lyngfelt, Leckner [92] and Adánez, de Diego [90]. The IGCC’s CO2 separation is based on pre-combustion, but Erlach, Schmidt [77] found chemical looping combustion to have a 2.8% higher net plant efficiency than the former method. Figure 3 illustrates the basic CLC system.
Table 3. An overview of chemical looping combustion-based carbon capture.
| Fuel Type | Operating Conditions P, T, C, G | Reactive Separator |
CC (%), Purity (%) | Challenges | Kinetics/Mass Transfer |
Ref. |
|---|---|---|---|---|---|---|
| Coal, C2H5OH, Isooctane, C3H8 and CH4. | T: 200–1200; molar ratios of carbon/CaSO4 = 0.5 and carbon/steam = 1 | TGA | NA, 93 (with CaSO4 at 850–975 °C) | The ΔHr is dependent on the fuel but not the amount of OC utilized. The yield depends on OC. | Combustion of iso-octane (−5101.58 kJ/mol) with Na2SO4 and CaSO4 produces without SO2 formation between 200 °C and 344.3 °C. | [93][94] |
| Syngas, H2 | XOC: 80–95, HR: 90–99, T: 370–1030 | 2-stage PBR- CLC | 100, NA | PP of O2 in reactors; high solid inventories. | The packed bed of OC reduces the need for highly efficient cyclone to reduce costs; boron nitride (BN) used as the dense support material due to high thermal conductivity, low thermal expansion and high thermal stability. | [95][96][97][98] |
| Coal, kerosene, biomass | Bd: 4.750; Dp: 128 Umf: 0.0129, Φ: 0.64 | IFBR | 83–99.3% at 800–950 °C, NA | Scale-up, fuel conversion, agglomeration and attrition. | ΔPRC increases linearly with solid flow rate. | [99][100][101][102][103][104][105] |
| CH4, coal | Iron oxide: 950 °C, FF: 1.18, CO2 EF: 10, DT: 5.25 | CMBS or RPBR (1 MWth) | >99, >95 | Reaction heat exceeds the convective heat-transfer rate to the gas flow. | The reduction kinetics and activation energy parameters are critical to find fuel conversion efficiency, temperature distribution and carbon separation efficiency. | [106][107] |
| CH4, syngas | T: 700–975; SITC:20–30; SFRR: 8–10 for CO SFRR:4–12 for H2 Fsolids:1.7–2.5 | CC-MBR | >99% CH4 and 100% syngas conversion. >99.99% H2 purity. | The formation of FeO and FeAl2O4 indicates further utilization of oxygen in iron-based OC׳s can be achieved.–ϕ > 1.14. | At 900 °C, the reduction of Fe2O3 to Fe with CO generates 37.7 kJ/mol Fe2O3 of heat but its reduction with H2 gas needs 61.8 kJ/mol Fe2O3 of heat. | [107][108] |
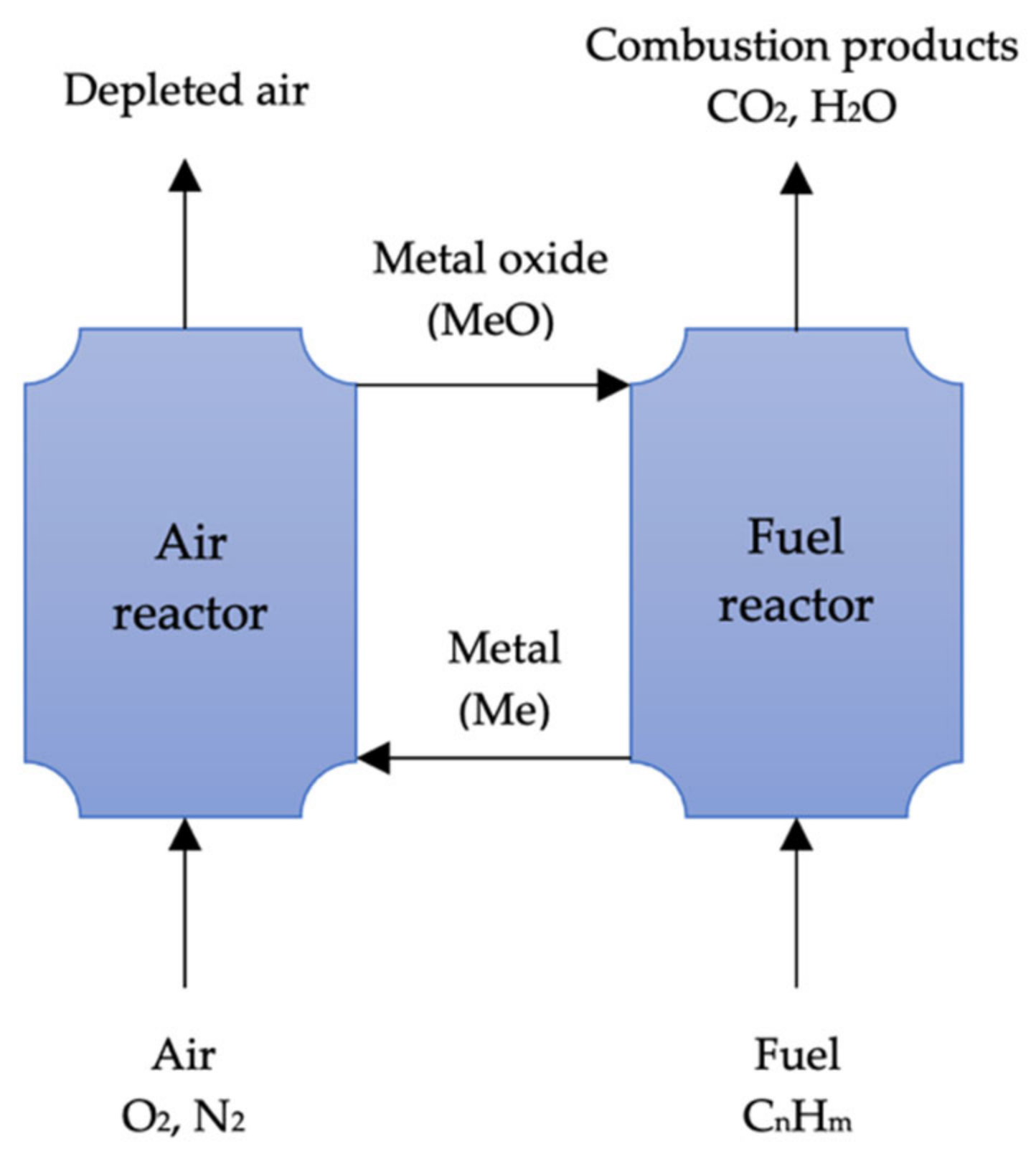
Figure 3. Schematic of chemical-looping combustion (adapted from Yang [109]).
4. Membrane Separation
Membrane separation uses a semipermeable membrane or barrier to physically separate CO2 from other flue gases [110]. Membrane separation uses less energy than traditional solvent absorption methods, making it less expensive [111]. Membrane separation has successfully been used for selective gas separation in a variety of fields for the past two decades, including natural gas sweetening, air separation, hydrogen production, and biogas upgrading. Researchers are working on developing membrane-based materials to separate CO2 released by various industries. Furthermore, this technology has produced increased efficiency in terms of both the economy and the environment [112]. Scientists have developed a variety of different membranes for CO2 separation, including inorganic membranes, polymers, carbon molecular sieve membranes (CMSMs), microporous organic polymers (MOPs), and mixed matrix membranes (MMMs) [113]. The potential sorbents and processes of membrane separation are highlighted in Table 4.
In addition, membrane separation technology can also separate gases in CCS processes such as pre- and post-combustion capture. It is generally considered that polymeric membranes are more flexible, durable, and efficient at capturing CO2 from industrial processes. An upper bound relationship analysis describes how selectivity and permeability are related to CO2 capture by polymeric membranes [114]. To improve results, glassy and rubbery materials with varying separation principles based on their size and diffusion ability can be used to synthesize polymeric membranes. The condensability and differences in kinetic properties of gas molecules are responsible for gas separation by glassy and rubbery polymers [115]. Considering how difficult it is to examine operating conditions for rapid performance, membranes applied in gas separation systems are typically modeled to determine their working capacity [116]. For optimal results in industrial settings, membrane performance must not be interfered with by flue gas impurities [113]. Researchers were able to separate CO2 from other gases with an efficiency of 82–88% [117][118]. In fact, despite membrane materials having poor permeability and selectivity [119], it is also problematic to use this extraction method in flue gas with low pressure and CO2 concentration in flue gas conditions [120]. A membrane carbon capture process is displayed in Figure 4.
Table 4. Summary of membrane-based carbon capture.
| Membrane | Reactive Separator | Operating Parameters | Challenges | Kinetics/Mass Transfer |
Ref. |
|---|---|---|---|---|---|
| Dense membranes | Hollow fiber and flat-sheet | S-P, T, P, La, pressure ratio of the permeate side to the feed side, pore size and porosity | Lower selectivity at higher permeability | Solution–diffusion; among the mechanisms are Knudsen diffusion and the molecular sieve effect | [121] |
| Micro-porous Membranes | Hollow fiber and flat-sheet | P, T, pore size and ε of the membrane–membrane wettability | Wetting of the membrane | Reaction kinetics depend on solvent | [121] |
| Gas flow area | There are other compounds present in the gas stream | Even at high pressures, Ko is controlled by the resistance of the liquid film | [122] | ||
| Liquid flow area | Solvent volatility and limited long-term stability | Pore diffusion depends on membrane support | [123] | ||
| Liquid in the membrane pores | Flat-sheet only | Ga, La, VVIS, P, T | Solvent “wash-out” causes the membrane’s stability to decrease | The overall mass transfer coefficient | [124] |
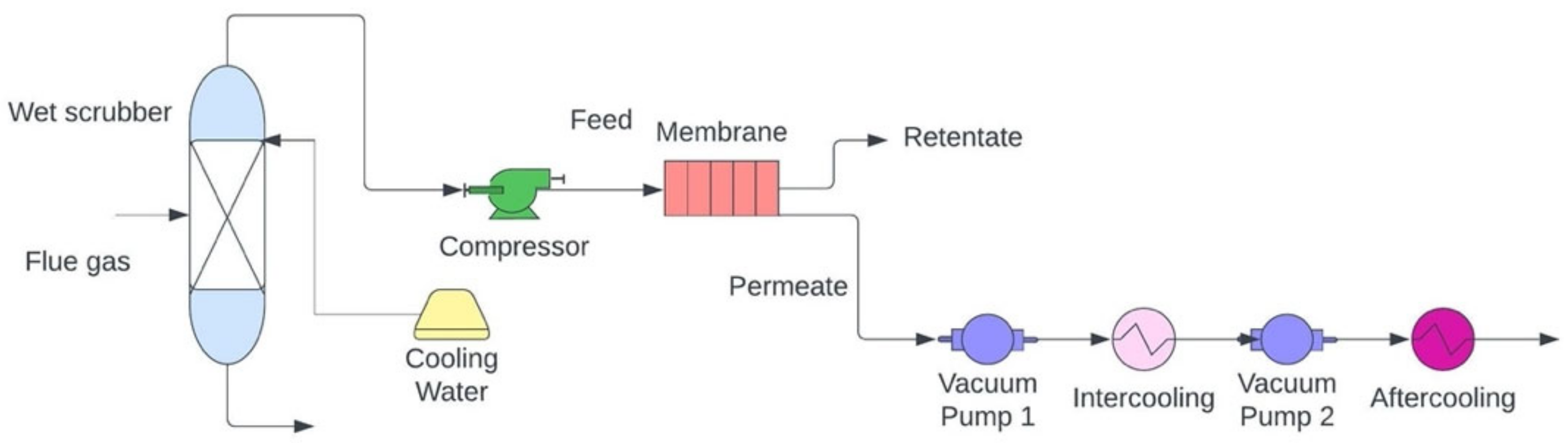
Figure 4. Schematic of membrane carbon capture process (adapted from Wang [125]).
5. Cryogenic Distillation
This process separates CO2 from gas mixtures by focusing on their boiling points at temperatures ranging from 100 to 135 °C [26][126]. In the presence of high pressures (100–200 atm), solidified CO2 provides two significant benefits: a lack of solvents and liquefied CO2 for more convenient transport and injection [127]. It does, however, have some drawbacks that need to be investigated further, as do other processes. When cold and pressurized nitrogen is used as a refrigerant, ice formation compromises equipment safety, causing pressure fluctuations and pipe blockages, as well as increasing the consumption of energy [127][128]. This enhanced CO2 separation can nullify the need for refrigerant preparation and storage [128][129]. However, CO2 is separated using cryogenic distillation coupled with biogas upgrading. A comparison of different separation methods for CO2 capture is shown in Table 5.
| Parameter | Chemical Absorption |
Physical Absorption |
Adsorption | Chemical-Looping Combustion |
Membrane Separation |
Cryogenic |
|---|---|---|---|---|---|---|
| Separation technique | Amine, chilled ammonia, and amino acid salt solvent. | Rectisol, Selexol, etc. Mostly integrated gasification combined cycle. | Pressure swing adsorption and pressure–temperature swing adsorption. | FeO, CuO, MnO, and NiO | Polymeric, inorganic and mixed membranes. | Cryogenic distillation. |
| Pros | High reactivity, low cost of the solvent, and low molecular weight result in a high mass-based absorption capacity, and moderate thermal stability and thermal degradation rate. | Highly recommended for separating CO2 during pre-combustion processes that operate at elevated CO2 partial pressures. Captures CO2 selectively from a gas stream without a chemical reaction |
Recycling is possible since it is a reversible process. It is possible to achieve high adsorption efficiency (485%). Low waste generation. |
Very high CO2 concentration. Low-cost oxygen carrier materials. Truly and directly reduces the atmospheric CO2 concentration. Viable alternative for CO2 capture from mobile and decentralized sources. |
No regeneration processes. Less solid waste produced. Less chemical consumption. High efficiency (>95% for single metal). |
High capture efficiency (up to 99.9%). Mature technology. For many years, CO2 has been recovered in the industry by this method. |
| Cons | Relatively high maintenance cost. | High energy is required to compress feed gas to a high pressure. Low CO2 solubility. Less efficient absorption process. Large equipment sizing. |
Requires adsorbent capable of operating at elevated temperatures. The significant amount of energy needed for CO2 desorption is high. |
Currently, the process is under development, and large-scale operations have not yet been carried out. | Fouling and low fluxes are examples of operational issues. High running costs. Removal (%) decreases with the presence of other metals. |
High energy requirement due to refrigeration. High capital expenditure. Need for removal of water, NOx, SOx, and other trace components to avoid the freezing and eventual blockage of process equipment. The procedure consumes a significant amount of energy. |
| CO2 concentration (vol.%) | <30.4 | >59.3 | 28–34 | 3–8 | 11.8 | <90 |
| CO2 capture efficiency (%) | 95 | >90 | <85 | 52–60 | 90 | 99.9 |
| CO2 capture cost (USD/tonne CO2) | 26.2 | 25.1 | 6.94 | 16–26 | 3–10 | 32.7 |
| CO2 purity (%) | 99 | <99 | 99.98 | >96 | 95 | 99.95 |
| Status of research and development | SaskPower, Saskatchewan, Canada (Boundary Dam Carbon Capture Project) TransAlta Corporation, Alberta Canada (Project Pioneer Keephills 3 Power Plant) American Electric Power, OH, USA (Mountaineer Power Plant) |
Summit Power Group, LLC, Seattle, USA (Texas Clean Energy Project) Don valley, Yorkshire, UK (Don Valley Power Project) Nuon Power, Buggenum, The Netherlands (Integrated gasification combined cycle plant) |
Under developmental stage. | Less large-scale demonstration plants. | Schwarze Pumpe power station, Spremberg, Germany (Oxy-fuel technology) CS Energy: Callide Power Plant A, Queensland, Australia (Callide Oxy-fuel Project) OxyCoal, UK (Oxy-fuel technology) |
Air Products and Chemicals, Inc., Pennsylvania USA |
This entry is adapted from the peer-reviewed paper 10.3390/atmos13121958
References
- Liu, Y.; Fan, W.; Wang, K.; Wang, J. Studies of CO2 absorption/regeneration performances of novel aqueous monothanlamine (MEA)-based solutions. J. Clean. Prod. 2016, 112, 4012–4021.
- Liu, Z.; Balasubramanian, R. Upgrading of waste biomass by hydrothermal carbonization (HTC) and low temperature pyrolysis (LTP): A comparative evaluation. Appl. Energy 2014, 114, 857–864.
- Yeh, A.C.; Bai, H. Comparison of ammonia and monoethanolamine solvents to reduce CO2 greenhouse gas emissions. Sci. Total Environ. 1999, 228, 121–133.
- Diao, Y.-F.; Zheng, X.-Y.; He, B.-S.; Chen, C.-H.; Xu, X.-C. Experimental study on capturing CO2 greenhouse gas by ammonia scrubbing. Energy Convers. Manag. 2004, 45, 2283–2296.
- Sreenivasulu, B.; Gayatri, D.; Sreedhar, I.; Raghavan, K. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350.
- Russo, M.; Olivieri, G.; Marzocchella, A.; Salatino, P.; Caramuscio, P.; Cavaleiro, C. Post-combustion carbon capture mediated by carbonic anhydrase. Sep. Purif. Technol. 2013, 107, 331–339.
- Kumar, N.; Rao, D.P. Design of a packed column for absorption of carbon dioxide in hot K2CO3 solution promoted by arsenious acid. Gas Sep. Purif. 1989, 3, 152–155.
- Derks, P.; Kleingeld, T.; van Aken, C.; Hogendoorn, J.; Versteeg, G. Kinetics of absorption of carbon dioxide in aqueous piperazine solutions. Chem. Eng. Sci. 2006, 61, 6837–6854.
- Guo, H.; Zhou, Z.; Jing, G. Kinetics of carbon dioxide absorption into aqueous solution. Int. J. Greenh. Gas Control 2013, 16, 197–205.
- Wappel, D.; Gronald, G.; Kalb, R.; Draxler, J. Ionic liquids for post-combustion CO2 absorption. Int. J. Greenh. Gas Control 2010, 4, 486–494.
- Rogers, R.D. Reflections on ionic liquids. Nature 2007, 447, 917–918.
- Davis, J.H., Jr. Task-Specific Ionic Liquids for Separations of Petrochemical Relevance: Reactive Capture of CO2 Using Amine Incorporating Ions; ACS Publications: Washnigton, DC, USA, 2005.
- Rahimpour, M.; Kashkooli, A. Enhanced carbon dioxide removal by promoted hot potassium carbonate in a split-flow absorber. Chem. Eng. Process. Process Intensif. 2004, 43, 857–865.
- Lin, P.-H.; Wong, D.S.H. Carbon dioxide capture and regeneration with amine/alcohol/water blends. Int. J. Greenh. Gas Control 2014, 26, 69–75.
- Hoffman, J.S.; Hammache, S.; Gray, M.L.; Fauth, D.J.; Pennline, H.W. Parametric study for an immobilized amine sorbent in a regenerative carbon dioxide capture process. Fuel Process. Technol. 2014, 126, 173–187.
- Xu, Z.; Wang, S.; Chen, C. CO2 absorption by biphasic solvents: Mixtures of 1, 4-Butanediamine and 2-(Diethylamino)-ethanol. Int. J. Greenh. Gas Control 2013, 16, 107–115.
- Freeman, S.A.; Dugas, R.; van Wagener, D.H.; Nguyen, T.; Rochelle, G.T. Carbon dioxide capture with concentrated, aqueous piperazine. Int. J. Greenh. Gas Control 2010, 4, 119–124.
- Cheng, H.-H.; Tan, C.-S. Reduction of CO2 concentration in a zinc/air battery by absorption in a rotating packed bed. J. Power Sources 2006, 162, 1431–1436.
- Xu, G.; Zhang, C.; Qin, S.; Wang, Y. Kinetics study on absorption of carbon dioxide into solutions of activated methyldiethanolamine. Ind. Eng. Chem. Res. 1992, 31, 921–927.
- Notz, R.; Asprion, N.; Clausen, I.; Hasse, H. Selection and pilot plant tests of new absorbents for post-combustion carbon dioxide capture. Chem. Eng. Res. Des. 2007, 85, 510–515.
- Chen, X.; Closmann, F.; Rochelle, G.T. Accurate screening of amines by the wetted wall column. Energy Procedia 2011, 4, 101–108.
- Rochelle, G.; Chen, E.; Freeman, S.; van Wagener, D.; Xu, Q.; Voice, A. Aqueous piperazine as the new standard for CO2 capture technology. Chem. Eng. J. 2011, 171, 725–733.
- Adeosun, A.; Abbas, Z.; Abu-Zahra, M.R. Screening and characterization of advanced amine based solvent systems for CO2 post-combustion capture. Energy Procedia 2013, 37, 300–305.
- Dash, S.K.; Samanta, A.N.; Bandyopadhyay, S.S. Simulation and parametric study of post combustion CO2 capture process using (AMP+ PZ) blended solvent. Int. J. Greenh. Gas Control 2014, 21, 130–139.
- Edrisi, S.A.; Tripathi, V.; Dubey, P.K.; Abhilash, P. Carbon sequestration and harnessing biomaterials from terrestrial plantations for mitigating climate change impacts. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 299–313.
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The US Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20.
- Khurana, N.; Goswami, N.; Sarmah, R. Carbon Capture: Innovation for a Green Environment. In Advances in Carbon Capture and Utilization; Springer: Berlin/Heidelberg, Germany, 2021; pp. 11–31.
- Kashyap, N.; Deka, B.; Choudhari, B.; Singh, B.P.; Pachani, S. Present status of capture of carbon dioxide and its storage technologies: A review. Pharma Innov. J. 2019, 8, 327–332.
- Heldebrant, D.J.; Koech, P.K.; Glezakou, V.-A.; Rousseau, R.; Malhotra, D.; Cantu, D.C. Water-lean solvents for post-combustion CO2 capture: Fundamentals, uncertainties, opportunities, and outlook. Chem. Rev. 2017, 117, 9594–9624.
- Bishnoi, S.; Rochelle, G.T. Absorption of carbon dioxide into aqueous piperazine: Reaction kinetics, mass transfer and solubility. Chem. Eng. Sci. 2000, 55, 5531–5543.
- Austgen, D.M.; Rochelle, G.T.; Chen, C.C. Model of vapor-liquid equilibria for aqueous acid gas-alkanolamine systems. 2. Representation of H2S and CO2 solubility in aqueous MDEA and CO2 solubility in aqueous mixtures of MDEA with MEA or DEA. Ind. Eng. Chem. Res. 1991, 30, 543–555.
- Barzagli, F.; Mani, F.; Peruzzini, M. Continuous cycles of CO2 absorption and amine regeneration with aqueous alkanolamines: A comparison of the efficiency between pure and blended DEA, MDEA and AMP solutions by 13C NMR spectroscopy. Energy Environ. Sci. 2010, 3, 772–779.
- Bishnoi, S.; Rochelle, G.T. Physical and chemical solubility of carbon dioxide in aqueous methyldiethanolamine. Fluid Phase Equilibria 2000, 168, 241–258.
- Xu, G.-W.; Zhang, C.-F.; Qin, S.-J.; Gao, W.-H.; Liu, H.-B. Gas− liquid equilibrium in a CO2 − MDEA− H2O system and the effect of piperazine on it. Ind. Eng. Chem. Res. 1998, 37, 1473–1477.
- Aroonwilas, A.; Veawab, A. Integration of CO2 capture unit using single-and blended-amines into supercritical coal-fired power plants: Implications for emission and energy management. Int. J. Greenh. Gas Control 2007, 1, 143–150.
- Veawab, A.; Aroonwilas, A.; Tontiwachwuthikul, P. CO2 absorption performance of aqueous alkanolamines in packed columns. Fuel Chem. Div. Prepr. 2002, 47, 49–50.
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348.
- Knudsen, J.N.; Andersen, J.; Jensen, J.N.; Biede, O. Evaluation of process upgrades and novel solvents for the post combustion CO2 capture process in pilot-scale. Energy Procedia 2011, 4, 1558–1565.
- Gurkan, B.E.; de la Fuente, J.C.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brennecke, J.F. Equimolar CO2 absorption by anion-functionalized ionic liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117.
- Bougie, F.; Iliuta, M.C. CO2 absorption in aqueous piperazine solutions: Experimental study and modeling. J. Chem. Eng. Data 2011, 56, 1547–1554.
- Rochelle, G.T. Thermal degradation of amines for CO2 capture. Curr. Opin. Chem. Eng. 2012, 1, 183–190.
- Fredriksen, S.; Jens, K.-J. Oxidative degradation of aqueous amine solutions of MEA, AMP, MDEA, Pz: A review. Energy Procedia 2013, 37, 1770–1777.
- Kozak, F.; Petig, A.; Morris, E.; Rhudy, R.; Thimsen, D. Chilled ammonia process for CO2 capture. Energy Procedia 2009, 1, 1419–1426.
- Toftegaard, M.B.; Brix, J.; Jensen, P.A.; Glarborg, P.; Jensen, A.D. Oxy-fuel combustion of solid fuels. Prog. Energy Combust. Sci. 2010, 36, 581–625.
- Asif, M.; Suleman, M.; Haq, I.; Jamal, S.A. Post-combustion CO2 capture with chemical absorption and hybrid system: Current status and challenges. Greenh. Gases Sci. Technol. 2018, 8, 998–1031.
- Tan, J.; Shao, H.; Xu, J.; Du, L.; Luo, G. Mixture absorption system of monoethanolamine-triethylene glycol for CO2 capture. Ind. Eng. Chem. Res. 2011, 50, 3966–3976.
- Rivas, O.; Prausnitz, J. Sweetening of sour natural gases by mixed-solvent absorption: Solubilities of ethane, carbon dioxide, and hydrogen sulfide in mixtures of physical and chemical solvents. AIChE J. 1979, 25, 975–984.
- Barzagli, F.; Di Vaira, M.; Mani, F.; Peruzzini, M. Improved Solvent Formulations for Efficient CO2 Absorption and Low-Temperature Desorption. ChemSusChem 2012, 5, 1724–1731.
- Liu, A.H.; Li, J.J.; Ren, B.H.; Lu, X.B. Development of High-Capacity and Water-Lean CO2 Absorbents by a Concise Molecular Design Strategy through Viscosity Control. ChemSusChem 2019, 12, 5164–5171.
- Li, X.; Liu, J.; Jiang, W.; Gao, G.; Wu, F.; Luo, C.; Zhang, L. Low energy-consuming CO2 capture by phase change absorbents of amine/alcohol/H2O. Sep. Purif. Technol. 2021, 275, 119181.
- Shen, L.; Liu, F.; Shen, Y.; Sun, C.; Zhang, Y.; Wang, Q.; Li, S.; Li, W. Novel biphasic solvent of AEP/1-propanol/H2O for CO2 capture with efficient regeneration performance and low energy consumption. Sep. Purif. Technol. 2021, 270, 118700.
- Karlsson, H.K.; Makhool, H.; Karlsson, M.; Svensson, H. Chemical absorption of carbon dioxide in non-aqueous systems using the amine 2-amino-2-methyl-1-propanol in dimethyl sulfoxide and N-methyl-2-pyrrolidone. Sep. Purif. Technol. 2021, 256, 117789.
- Alkhatib, I.I.; Khalifa, O.; Bahamon, D.; Abu-Zahra, M.R.; Vega, L.F. Sustainability criteria as a game changer in the search for hybrid solvents for CO2 and H2S removal. Sep. Purif. Technol. 2021, 277, 119516.
- Mathias, P.M.; Afshar, K.; Zheng, F.; Bearden, M.D.; Freeman, C.J.; Andrea, T.; Koech, P.K.; Kutnyakov, I.; Zwoster, A.; Smith, A.R. Improving the regeneration of CO2-binding organic liquids with a polarity change. Energy Environ. Sci. 2013, 6, 2233–2242.
- Barzagli, F.; Giorgi, C.; Mani, F.; Peruzzini, M. Screening study of different amine-based solutions as sorbents for direct CO2 capture from air. ACS Sustain. Chem. Eng. 2020, 8, 14013–14021.
- Wanderley, R.R.; Pinto, D.D.; Knuutila, H.K. From hybrid solvents to water-lean solvents–A critical and historical review. Sep. Purif. Technol. 2021, 260, 118193.
- Liu, F.; Shen, Y.; Shen, L.; Zhang, Y.; Chen, W.; Wang, Q.; Li, S.; Zhang, S.; Li, W. Sustainable ionic liquid organic solution with efficient recyclability and low regeneration energy consumption for CO2 capture. Sep. Purif. Technol. 2021, 275, 119123.
- Wai, S.K.; Nwaoha, C.; Saiwan, C.; Idem, R.; Supap, T. Absorption heat, solubility, absorption and desorption rates, cyclic capacity, heat duty, and absorption kinetic modeling of AMP–DETA blend for post–combustion CO2 capture. Sep. Purif. Technol. 2018, 194, 89–95.
- Muchan, P.; Narku-Tetteh, J.; Saiwan, C.; Idem, R.; Supap, T. Effect of number of amine groups in aqueous polyamine solution on carbon dioxide (CO2) capture activities. Sep. Purif. Technol. 2017, 184, 128–134.
- Singto, S.; Supap, T.; Idem, R.; Tontiwachwuthikul, P.; Tantayanon, S.; Al-Marri, M.J.; Benamor, A. Synthesis of new amines for enhanced carbon dioxide (CO2) capture performance: The effect of chemical structure on equilibrium solubility, cyclic capacity, kinetics of absorption and regeneration, and heats of absorption and regeneration. Sep. Purif. Technol. 2016, 167, 97–107.
- Zhou, T.; McBride, K.; Linke, S.; Song, Z.; Sundmacher, K. Computer-aided solvent selection and design for efficient chemical processes. Curr. Opin. Chem. Eng. 2020, 27, 35–44.
- Malhotra, D.; Koech, P.K.; Heldebrant, D.J.; Cantu, D.C.; Zheng, F.; Glezakou, V.A.; Rousseau, R. Reinventing design principles for developing low-viscosity carbon dioxide-binding organic liquids for flue gas clean up. ChemSusChem 2017, 10, 636–642.
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. SAFT: Equation-of-state solution model for associating fluids. Fluid Phase Equilibria 1989, 52, 31–38.
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. New reference equation of state for associating liquids. Ind. Eng. Chem. Res. 1990, 29, 1709–1721.
- Papadokonstantakis, S.; Badr, S.; Hungerbühler, K.; Papadopoulos, A.I.; Damartzis, T.; Seferlis, P.; Forte, E.; Chremos, A.; Galindo, A.; Jackson, G. Toward sustainable solvent-based postcombustion CO2 capture: From molecules to conceptual flowsheet design. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2015; Volume 36, pp. 279–310.
- Limleamthong, P.; Gonzalez-Miquel, M.; Papadokonstantakis, S.; Papadopoulos, A.I.; Seferlis, P.; Guillén-Gosálbez, G. Multi-criteria screening of chemicals considering thermodynamic and life cycle assessment metrics via data envelopment analysis: Application to CO2 capture. Green Chem. 2016, 18, 6468–6481.
- Papadopoulos, A.I.; Badr, S.; Chremos, A.; Forte, E.; Zarogiannis, T.; Seferlis, P.; Papadokonstantakis, S.; Adjiman, C.S.; Galindo, A.; Jackson, G. Efficient screening and selection of post-combustion CO2 capture solvents. Chem. Eng. 2014, 39, 211–216.
- Papadopoulos, A.I.; Badr, S.; Chremos, A.; Forte, E.; Zarogiannis, T.; Seferlis, P.; Papadokonstantakis, S.; Galindo, A.; Jackson, G.; Adjiman, C.S. Computer-aided molecular design and selection of CO2 capture solvents based on thermodynamics, reactivity and sustainability. Mol. Syst. Des. Eng. 2016, 1, 313–334.
- Mota-Martinez, M.T.; Hallett, J.P.; Mac Dowell, N. Solvent selection and design for CO2 capture–how we might have been missing the point. Sustain. Energy Fuels 2017, 1, 2078–2090.
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998.
- Tobiszewski, M.; Tsakovski, S.; Simeonov, V.; Namieśnik, J.; Pena-Pereira, F. A solvent selection guide based on chemometrics and multicriteria decision analysis. Green Chem. 2015, 17, 4773–4785.
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398.
- Spigarelli, B.P. A Novel Approach to Carbon Dioxide Capture and Storage. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2013.
- Ben-Mansour, R.; Habib, M.; Bamidele, O.; Basha, M.; Qasem, N.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations—A review. Appl. Energy 2016, 161, 225.
- Takamura, Y.; Aoki, J.; Uchida, S.; Narita, S. Application of high-pressure swing adsorption process for improvement of CO2 recovery system from flue gas. Can. J. Chem. Eng. 2001, 79, 812–816.
- Wyczalek, F. Energy Independence-A Nation Running on Empty. In Proceedings of the 3rd International Energy Conversion Engineering Conference, San Francisco, CA, USA, 15–18 August 2022; p. 5545.
- Erlach, B.; Schmidt, M.; Tsatsaronis, G. Comparison of carbon capture IGCC with pre-combustion decarbonisation and with chemical-looping combustion. Energy 2011, 36, 3804–3815.
- Clausse, M.; Merel, J.; Meunier, F. Numerical parametric study on CO2 capture by indirect thermal swing adsorption. Int. J. Greenh. Gas Control 2011, 5, 1206–1213.
- Kulkarni, A.R.; Sholl, D.S. Analysis of equilibrium-based TSA processes for direct capture of CO2 from air. Ind. Eng. Chem. Res. 2012, 51, 8631–8645.
- Wang, L.; Yang, Y.; Shen, W.; Kong, X.; Li, P.; Yu, J.; Rodrigues, A.E. Experimental evaluation of adsorption technology for CO2 capture from flue gas in an existing coal-fired power plant. Chem. Eng. Sci. 2013, 101, 615–619.
- Xu, D.; Xiao, P.; Zhang, J.; Li, G.; Xiao, G.; Webley, P.A.; Zhai, Y. Effects of water vapour on CO2 capture with vacuum swing adsorption using activated carbon. Chem. Eng. J. 2013, 230, 64–72.
- Yang, H.; Yuan, Y.; Tsang, S.C.E. Nitrogen-enriched carbonaceous materials with hierarchical micro-mesopore structures for efficient CO2 capture. Chem. Eng. J. 2012, 185, 374–379.
- Arenillas, A.; Smith, K.; Drage, T.; Snape, C. CO2 capture using some fly ash-derived carbon materials. Fuel 2005, 84, 2204–2210.
- Kim, K.; Son, Y.; Lee, W.B.; Lee, K.S. Moving bed adsorption process with internal heat integration for carbon dioxide capture. Int. J. Greenh. Gas Control 2013, 17, 13–24.
- Kumar, R. Adsorption column blowdown: Adiabatic equilibrium model for bulk binary gas mixtures. Ind. Eng. Chem. Res. 1989, 28, 1677–1683.
- An, H.; Feng, B.; Su, S. Effect of monolithic structure on CO2 adsorption performance of activated carbon fiber–phenolic resin composite: A simulation study. Fuel 2013, 103, 80–86.
- Ishida, M.; Yamamoto, M.; Ohba, T. Experimental results of chemical-looping combustion with NiO/NiAl2O4 particle circulation at 1200 C. Energy Convers. Manag. 2002, 43, 1469–1478.
- Cho, P.; Mattisson, T.; Lyngfelt, A. Reactivity of iron oxide with methane in a laboratory fluidized bed: Application of chemical-looping combustion. In Proceedings of the 7th International Conference on Circulating Fluidized Bed Technology, Niagara Falls, ON, Canada, 5–8 May 2002.
- Guo, Q.; Zhang, J.; Tian, H. Recent advances in CaSO4 oxygen carrier for chemical-looping combustion (CLC) process. Chem. Eng. Commun. 2012, 199, 1463–1491.
- Adánez, J.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Palacios, J. Selection of oxygen carriers for chemical-looping combustion. Energy Fuels 2004, 18, 371–377.
- Zafar, Q.; Mattisson, T.; Gevert, B. Integrated hydrogen and power production with CO2 capture using chemical-looping reforming redox reactivity of particles of CuO, Mn2O3, NiO, and Fe2O3 using SiO2 as a support. Ind. Eng. Chem. Res. 2005, 44, 3485–3496.
- Lyngfelt, A.; Leckner, B.; Mattisson, T. A fluidized-bed combustion process with inherent CO2 separation; application of chemical-looping combustion. Chem. Eng. Sci. 2001, 56, 3101–3113.
- Kale, G. Feasibility study of sulfates as oxygen carriers for chemical looping processes. QScience Connect 2012, 2012, 1.
- Zheng, M.; Shen, L.; Xiao, J. Reduction of CaSO4 oxygen carrier with coal in chemical-looping combustion: Effects of temperature and gasification intermediate. Int. J. Greenh. Gas Control 2010, 4, 716–728.
- Spallina, V.; Gallucci, F.; Romano, M.C.; Chiesa, P.; Lozza, G.; van Sint Annaland, M. Investigation of heat management for CLC of syngas in packed bed reactors. Chem. Eng. J. 2013, 225, 174–191.
- Hamers, H.; Gallucci, F.; Cobden, P.; Kimball, E.; van Sint Annaland, M. A novel reactor configuration for packed bed chemical-looping combustion of syngas. Int. J. Greenh. Gas Control 2013, 16, 1–12.
- Harper, R.N.; Boyce, C.M.; Scott, S.A. Oxygen carrier dispersion in inert packed beds to improve performance in chemical looping combustion. Chem. Eng. J. 2013, 234, 464–474.
- Cotton, A.; Patchigolla, K.; Oakey, J. Hydrodynamic characteristics of a pilot-scale cold model of a CO2 capture fluidised bed reactor. Powder Technol. 2013, 235, 1060–1069.
- Yazdanpanah, M.M.; Forret, A.; Gauthier, T.; Delebarre, A. An experimental investigation of loop-seal operation in an interconnected circulating fluidized bed system. Powder Technol. 2013, 237, 266–275.
- Moldenhauer, P.; Rydén, M.; Mattisson, T.; Lyngfelt, A. Chemical-looping combustion and chemical-looping with oxygen uncoupling of kerosene with Mn-and Cu-based oxygen carriers in a circulating fluidized-bed 300 W laboratory reactor. Fuel Process. Technol. 2012, 104, 378–389.
- Ku, Y.; Wu, H.-C.; Chiu, P.-C.; Tseng, Y.-H.; Kuo, Y.-L. Methane combustion by moving bed fuel reactor with Fe2O3/Al2O3 oxygen carriers. Appl. Energy 2014, 113, 1909–1915.
- Tong, A.; Sridhar, D.; Sun, Z.; Kim, H.R.; Zeng, L.; Wang, F.; Wang, D.; Kathe, M.V.; Luo, S.; Sun, Y. Continuous high purity hydrogen generation from a syngas chemical looping 25 kWth sub-pilot unit with 100% carbon capture. Fuel 2013, 103, 495–505.
- Wang, J.; Anthony, E.J. Clean combustion of solid fuels. Appl. Energy 2008, 85, 73–79.
- Jin, H.; Ishida, M. A new type of coal gas fueled chemical-looping combustion. Fuel 2004, 83, 2411–2417.
- Cao, Y.; Pan, W.-P. Investigation of chemical looping combustion by solid fuels. 1. Process analysis. Energy Fuels 2006, 20, 1836–1844.
- Adánez-Rubio, I.; Abad, A.; Gayán, P.; de Diego, L.; García-Labiano, F.; Adánez, J. Biomass combustion with CO2 capture by chemical looping with oxygen uncoupling (CLOU). Fuel Process. Technol. 2014, 124, 104–114.
- Gayán, P.; Abad, A.; de Diego, L.; García-Labiano, F.; Adánez, J. Assessment of technological solutions for improving chemical looping combustion of solid fuels with CO2 capture. Chem. Eng. J. 2013, 233, 56–69.
- Adánez-Rubio, I.; Abad, A.; Gayán, P.; de Diego, L.; García-Labiano, F.; Adánez, J. Performance of CLOU process in the combustion of different types of coal with CO2 capture. Int. J. Greenh. Gas Control 2013, 12, 430–440.
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27.
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300.
- Ansaloni, L.; Salas-Gay, J.; Ligi, S.; Baschetti, M.G. Nanocellulose-based membranes for CO2 capture. J. Membr. Sci. 2017, 522, 216–225.
- Li, X.; Cheng, Y.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H. Efficient CO2 capture by functionalized graphene oxide nanosheets as fillers to fabricate multi-permselective mixed matrix membranes. ACS Appl. Mater. Interfaces 2015, 7, 5528–5537.
- He, X.; Fu, C.; Hägg, M.-B. Membrane system design and process feasibility analysis for CO2 capture from flue gas with a fixed-site-carrier membrane. Chem. Eng. J. 2015, 268, 1–9.
- Turi, D.M.; Ho, M.; Ferrari, M.-C.; Chiesa, P.; Wiley, D.; Romano, M.C. CO2 capture from natural gas combined cycles by CO2 selective membranes. Int. J. Greenh. Gas Control 2017, 61, 168–183.
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450.
- Lee, P.S.; Lim, M.S.; Park, A.; Park, H.; Nam, S.-E.; Park, Y.-I. A zeolite membrane module composed of SAPO-34 hollow fibers for use in fluorinated gas enrichment. J. Membr. Sci. 2017, 542, 123–132.
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125.
- Favre, E. Membrane processes and postcombustion carbon dioxide capture: Challenges and prospects. Chem. Eng. J. 2011, 171, 782–793.
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663.
- Wang, L.; Boutilier, M.S.; Kidambi, P.R.; Jang, D.; Hadjiconstantinou, N.G.; Karnik, R. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes. Nat. Nanotechnol. 2017, 12, 509–522.
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628.
- Luis, P.; van Gerven, T.; van der Bruggen, B. Recent developments in membrane-based technologies for CO2 capture. Prog. Energy Combust. Sci. 2012, 38, 419–448.
- Paul, S.; Ghoshal, A.K.; Mandal, B. Theoretical studies on separation of CO2 by single and blended aqueous alkanolamine solvents in flat sheet membrane contactor (FSMC). Chem. Eng. J. 2008, 144, 352–360.
- Andersen, A.; Divekar, S.; Dasgupta, S.; Cavka, J.H.; Nanoti, A.; Spjelkavik, A.; Goswami, A.N.; Garg, M.; Blom, R. On the development of Vacuum Swing adsorption (VSA) technology for post-combustion CO2 capture. Energy Procedia 2013, 37, 33–39.
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A review of post-combustion CO2 capture technologies from coal-fired power plants. Energy Procedia 2017, 114, 650–665.
- Gimžauskaitė, D.; Aikas, M.; Tamošiūnas, A. Recent progress in thermal plasma gasification of liquid and solid wastes. Recent Adv. Renew. Energy Technol. 2022, 2, 155–196.
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443.
- Knapik, E.; Kosowski, P.; Stopa, J. Cryogenic liquefaction and separation of CO2 using nitrogen removal unit cold energy. Chem. Eng. Res. Des. 2018, 131, 66–79.
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Cryogenic technology for biogas upgrading combined with carbon capture-a review of systems and property impacts. Energy Procedia 2017, 142, 3741–3746.
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663.
- Damen, K.; van Troost, M.; Faaij, A.; Turkenburg, W. A comparison of electricity and hydrogen production systems with CO2 capture and storage. Part A: Review and selection of promising conversion and capture technologies. Prog. Energy Combust. Sci. 2006, 32, 215–246.
- Rubin, E.S.; Rao, A.B.; Chen, C. Comparative Assessments of Fossil Fuel Power Plants with CO. In Proceedings of the 7th International Conference on Greenhouse Gas Control Technologies, Vancouver, BC, Canada, 5–9 September 2004.
- Porter, R.T.; Fairweather, M.; Pourkashanian, M.; Woolley, R.M. The range and level of impurities in CO2 streams from different carbon capture sources. Int. J. Greenh. Gas Control 2015, 36, 161–174.
- Kanniche, M.; Le Moullec, Y.; Authier, O.; Hagi, H.; Bontemps, D.; Neveux, T.; Louis-Louisy, M. Up-to-date CO2 capture in thermal power plants. Energy Procedia 2017, 114, 95–103.
- Dillon, D.; White, V.; Allam, R.; Wall, R.; Gibbins, J. Oxy Combustion Processes for CO2 Capture from Power Plant. In Technology & Engineering; Engineering Investigation Report; Mitsui Babcock Energy Limited: Crawley, UK, 2005; Volume 9.
- Zheng, L. Oxy-Fuel Combustion for Power Generation and Carbon Dioxide (CO2) Capture; Elsevier: Amsterdam, The Netherlands, 2011.
- Bhown, A.S.; Freeman, B.C. Analysis and status of post-combustion carbon dioxide capture technologies. Environ. Sci. Technol. 2011, 45, 8624–8632.
- Hendriks, C. Energy Conversion: CO2 Removal from Coal-Fired Power Plant; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995.
- Ritter, J.A. Radically new adsorption cycles for carbon dioxide sequestration. In Proceedings of the University Coal Research Contractors Review Meeting, U.S. DOE National Energy Technology Laboratory, Pittsburgh, PA, USA, 10 June 2004; Volume 2, p. 2.
- Olabi, A.; Obaideen, K.; Elsaid, K.; Wilberforce, T.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renew. Sustain. Energy Rev. 2022, 153, 111710.
- Lyngfelt, A.; Linderholm, C. Chemical-looping combustion of solid fuels–technology overview and recent operational results in 100 kW unit. Energy Procedia 2014, 63, 98–112.
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; Luis, F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282.
- Rackley, S.A. Carbon Capture and Storage; Butterworth-Heinemann: Oxford, UK, 2017.
- Gielen, D. The energy policy consequences of future CO2 capture and sequestration technologies. In Proceedings of the 2nd Annual Conference on Carbon Sequestration, Alexandria, VA, USA, 5–8 May 2003; pp. 5–8.
- Audus, H. Leading options for the capture of CO2 at power stations. In Proceedings of the 5th International Conference on Greenhouse Gas Control Technologies, Cairns, Australia, 13–16 August 2000; p. 16.
- Göttlicher, G.; Pruschek, R. Comparison of CO2 removal systems for fossil-fuelled power plant processes. Energy Convers. Manag. 1997, 38, S173–S178.
- Tuinier, M.; van Sint Annaland, M.; Kramer, G.J.; Kuipers, J. Cryogenic CO2 capture using dynamically operated packed beds. Chem. Eng. Sci. 2010, 65, 114–119.
- Zhang, X.; Song, Z.; Gani, R.; Zhou, T. Comparative economic analysis of physical, chemical, and hybrid absorption processes for carbon capture. Ind. Eng. Chem. Res. 2020, 59, 2005–2012.
- Li, J.; Zhang, H.; Gao, Z.; Fu, J.; Ao, W.; Dai, J. CO2 capture with chemical looping combustion of gaseous fuels: An overview. Energy Fuels 2017, 31, 3475–3524.
- Castel, C.; Bounaceur, R.; Favre, E. Membrane processes for direct carbon dioxide capture from air: Possibilities and limitations. Front. Chem. Eng. 2021, 3, 668867.
- Zhai, H.; Rubin, E.S. The effects of membrane-based CO2 capture system on pulverized coal power plant performance and cost. Energy Procedia 2013, 37, 1117–1124.
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102.
- Lyngfelt, A.; Leckner, B. A 1000 MWth boiler for chemical-looping combustion of solid fuels–Discussion of design and costs. Appl. Energy 2015, 157, 475–487.
- Moldenhauer, P.; Linderholm, C.; Rydén, M.; Lyngfelt, A. Avoiding CO2 capture effort and cost for negative CO2 emissions using industrial waste in chemical-looping combustion/gasification of biomass. Mitig. Adapt. Strateg. Glob. Change 2020, 25, 1–24.
This entry is offline, you can click here to edit this entry!
