Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Curcumin (diferuloylmethane)—the “golden spice”—has been widely studied because of its pleiotropic effects in cancer. Curcumin, a hydrophobic polyphenol, is derived from the rhizome of the herb Curcuma longa and constitutes the major curcuminoid in the spice turmeric (77% curcumin, 17% demethoxycurcumin, 3% bis-demethoxycurcumin). Curcumin is “generally recognized as safe” (GRAS) as a dietary supplement by the U.S Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) and has been catalogued with the E100 code of the European Union.
- curcumin
- metastatic colorectal cancer
- chemotherapy
1. Curcumin Attenuates Chemotherapy-Related Toxicity
For many years, curcumin (diferuloylmethane)—the “golden spice”—has been widely studied because of its pleiotropic effects in cancer. Curcumin, a hydrophobic polyphenol, is derived from the rhizome of the herb Curcuma longa and constitutes the major curcuminoid in the spice turmeric (77% curcumin, 17% demethoxycurcumin, 3% bis-demethoxycurcumin). Curcumin is “generally recognized as safe” (GRAS) as a dietary supplement by the U.S Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) and has been catalogued with the E100 code of the European Union. One of the clinical benefits of curcumin is the improvement of QoL in several health conditions [1], including cancer [2][3].
Curcumin is a pleiotropic agent that acts through multiple cellular pathways and has been shown to possess anti-cancer properties against CRC in vitro and in vivo [4][5]. Many of its anti-cancer properties have been attributed to its role as an anti-inflammatory and antioxidant, as well as to its ability to modulate the cell cycle and the pathways involved in proliferation, apoptosis, migration, invasion, angiogenesis, and metastasis [6], which are typically targeted by the drugs used to treat CRC. Mechanistically, curcumin modulates several CRC molecular targets at the same time—either by altering their gene expression, activation, or signaling pathways, or by direct interaction [4][5][6]. Importantly, in addition to its well-known anti-cancer properties, curcumin can also alleviate some of the chemotherapy-related side effects [7]. For example, curcumin attenuates the liver injury induced by oxaliplatin through activation of the nuclear factor-erythroid 2-related factor 2 (Nrf2) signaling, a key regulator pathway of cellular defense against oxidative and electrophilic stresses [8], as well as the nerve damage and the oxidative damage to mitochondria caused by oxaliplatin [9]. In fact, curcumin has been shown to not only hinder mitochondrial damage but also to protect mitochondria and induce activity of mitochondrial complex enzymes [3][9][10]. Interestingly, similar effects of curcumin on cisplatin-related toxicity have been observed in several tumor types [11][12][13][14][15]. Additionally, curcumin protects against irinotecan-induced intestinal injury by inhibiting nuclear factor kappa B (NF-κB) transcription factor activation [16], and it is also active against FOLFIRI-related cardiovascular toxicity [17] and capecitabine-induced hand-foot syndrome [18]. Recently, it has been shown that curcumin attenuates bevacizumab-associated cardiotoxicity by suppressing oxidative stress and preventing mitochondrial dysfunction in heart mitochondria [19].
In a study of curcumin’s effects in cancer patients, Belcaro and colleagues looked at the side effects of chemotherapy in several tumor types, including colon, ovarian, lung, liver, kidney, and stomach cancers. Of 80 patients treated with chemotherapy, 40 simultaneously received 500 mg of curcumin. Chemotherapy-related nausea, diarrhea, constipation, weight loss, neutropenia, and cardiotoxicity were significantly lower in the patients receiving curcumin than in the control group. Moreover, patients receiving curcumin also required fewer medications for treating these side effects [20]. In the same vein, turmeric supplementation for 21 days resulted in a clinically relevant and statistically significant improvement in global health status, symptom scores (fatigue, nausea, vomiting, pain, appetite loss, insomnia), and hematological parameters of breast cancer patients treated with paclitaxel [21]. Taken together, these findings lead researchers to suggest that the addition of curcumin to the standard treatment of CRC could not only attenuate chemotherapy-associated side effects but also improve the QoL of patients (Figure 1).
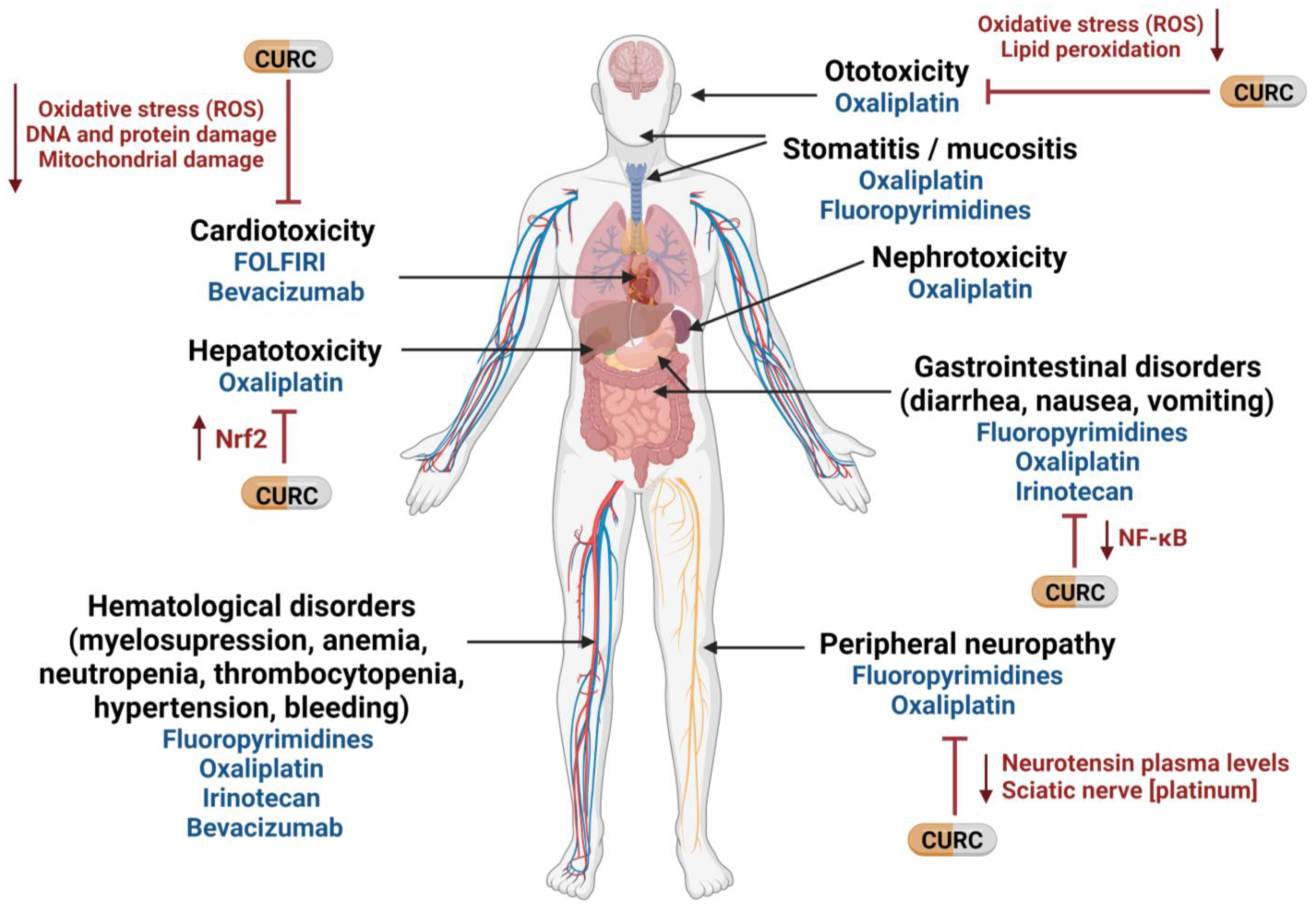
Figure 1. Potential use of curcumin to mitigate therapy-related side effects in mCRC. CURC: curcumin; NF-κB: nuclear factor kappa B; Nrf2: nuclear factor -erythroid 2- related factor 2; ROS: reactive oxygen species. Created with BioRender.com (accessed on 16 September 2022).
2. Curcumin Reverts Chemotherapy Resistance in mCRC
In addition to chemotherapy-related toxicity, chemoresistance remains one of the main problems hindering treatment success. Tumor cells can be intrinsically resistant or acquire resistance during a treatment. Resistance to chemotherapy is a complex and multifactorial process involving several mechanisms, including drug influx/efflux modifications, alterations in DNA damage repair (DDR), decreased cell death activation, autocrine survival signaling, and high detoxification activity [22][23]. One of these mechanisms with consequences in mCRC is the hyperactivation of the NF-κB signaling pathway [24], which promotes the expression of several target genes involved in inflammation, cell proliferation, apoptosis, angiogenesis, invasion, metastasis, and chemoresistance [25][26]. In fact, most of the anti-inflammatory and anti-cancer properties of curcumin are believed to be due to its ability to inhibit NF-κB activation through interaction with the IκB kinase complex (IKK) by inhibiting the phosphorylation and degradation of IκBα, a NF-κB inhibitor, and thereby blocking the nuclear translocation of this transcription factor [4][27][28]. Along with other studies [29][30][31], researchers demonstrated that curcumin can overcome oxaliplatin resistance by inhibiting the activity of the CXC-chemokines/NF-κB axis and, consequently, the expression of genes involved in anti-apoptosis and proliferation [24]. Additionally, in CRC preclinical models, curcumin was shown to enhance the effect of 5-fluorouracil [32][33] and capecitabine [34] by inhibiting AKT and NF-κB activity, and consequently, NF-κB-regulated gene products. In the same vein, Pattel and colleagues reported that curcumin sensitizes CRC cells to FOLFOX by inhibiting EGFR family receptors and insulin-like growth factor-1 receptor (IGF-1R) [35][36][37], the overexpression of which has been related to chemoresistance in CRC [38][39].
Chemotherapy resistance is also related to the specific mechanism of action of the drug. An example of such a specific mechanism is gene amplification in TS in 5-fluorouracil treated patients [40] and upregulation of genes involved in DDR pathways, such as ERCC1 in oxaliplatin treated patients [41]. Interestingly, Rajitha and colleagues demonstrated that the inhibition of NF-κB translocation by curcumin or its analogs induces cell cycle arrest and downregulates TS in CRC cells [28]. Furthermore, curcumin was found to inhibit ERCC1 through its ability to modulate miR-409-3p, thereby overcoming oxaliplatin resistance in CRC cells [42].
Curcumin can also promote the activation of apoptotic pathways by increasing the generation of reactive oxygen species (ROS) [43]. In a recent work, Li and colleagues demonstrated that curcumin can reverse Nicotinamide N-methyltransferase-induced cell proliferation and 5-fluorouracil resistance through ROS generation and cell cycle arrest [44].
On the other hand, the drug-resistant phenotype is associated with the acquisition of mesenchymal features, and epithelial-to-mesenchymal transition (EMT) plays a key role in chemoresistance in CRC, mainly through the activation of the NF-κB and transforming growth factor β (TGF-β) pathways [45][46][47]. In fact, EMT was observed in chemotherapy-resistant CRC cell lines [24][48][49], while curcumin was able to revert this chemoresistance by downregulating EMT markers [50] through TGF-β/Smad2/3 signaling attenuation [51], by upregulating EMT suppressive miRNAs [52] or by downregulating the TET1-NKD2-WNT signaling pathway [53]. In addition, several studies have demonstrated that curcumin can sensitize colon cancer stem cells (CSC), a small subpopulation of cells within tumors capable of self-renewal, differentiation, and tumorigenicity [54], to 5-fluorouracil, FOLFOX and irinotecan, thereby preventing the emergence of chemoresistant CRC cells [37][55][56][57][58]. In this regard, a recent study has demonstrated that treatment of CRC organoids with a combination of amorphous curcumin (a compound with improved solubility and bioavailability) and oxaliplatin, 5-fluoroouracil, or irinotecan showed a synergistic activity through the inhibition of proliferation-related signals and CSC marker expression, in addition to arresting the ERK signaling pathway [59]. Along the same lines, Zheng and colleagues showed that low doses of curcumin promoted the sensitivity of CRC cells to 5-fluorouracil by downregulating phospho-ERK signaling [60].
Finally, several studies have shown that curcumin can increase the intracellular accumulation of oxaliplatin and 5-fluorouracil in CRC cells by downregulating the P-gp [42][61] and ATP-binding cassette transporter G2 (ABCG2) [37] drug-efflux transporters both at the mRNA and protein levels. Preclinical data have suggested that the expression of ATP-binding cassette (ABC) transporters, such as ABCC2 [62], ABCB4 [63], as well as the multidrug resistance protein 1 (MDR1, also known as P-glycoprotein or P-gp), which is encoded by ABCB1 [64][65], can confer resistance to chemotherapy. However, evidence that these transporters contribute to drug resistance in human tumors is sorely lacking [66] and the development of MDR1 as a therapeutic target has been unsuccessful [67]. It is important to highlight that although several studies have related the ABC transporters’ overexpression to platinum resistance [22][68][69], the association between oxaliplatin resistance and the MDR1 expression has shown unconvincing results. For instance, Ekblad and colleagues described an overexpression of this membrane transporter as a consequence of oxaliplatin resistance acquisition in vitro, although functional tests did not show any increase in ABCB1 transport activity in the oxaliplatin-resistant models compared with its parental cell lines [70]. Other studies have reported no association between these drug efflux pumps and the sensitivity to oxaliplatin in CRC clinical samples [71]. In the same vein, the ability of MDR1 to confer resistance to 5-fluorouracil and irinotecan has been demonstrated in different CRC cell lines transfected with this carrier. However, its clinical relevance in CRC refractoriness to antitumor chemotherapy remains to be established [72][73]. Taken together, these results highlight the necessity of further investigation into the role of MDR1 and curcumin in oxaliplatin and 5-fluorouracil resistance in CRC patients.
Most clinical data on curcumin come from early phase clinical trials, with results showing that oral curcumin can achieve efficacious levels in the colon with negligible distribution outside the gut [74][75]. Moreover, curcumin was shown to be safe in advanced CRC patients when administered for up to four months [76]. In addition, a study by James and colleagues found that curcumin at doses up to 2 gms daily was highly tolerable when added to a FOLFOX regimen in mCRC patients with liver metastases [77]. More recently, the same group performed a phase IIa randomized trial of first-line treatment for mCRC patients comparing FOLFOX +/−bevacizumab with the same regimen plus curcumin 2 gms/day (CUFOX) in mCRC patients. In the intention-to-treat population, patients in the CUFOX arm achieved longer overall survival (HR 0.34; p = 0.02) but there was no difference in progression-free survival (HR 0.57) [78].
In conclusion, a further improvement in outcomes for mCRC highly depends on identifying and targeting mechanisms of drug resistance. Taken together, these findings offer compelling evidence that combining curcumin with conventional chemotherapy may be effective in overcoming drug resistance in mCRC
This entry is adapted from the peer-reviewed paper 10.3390/ijms232214058
References
- Sadeghian, M.; Rahmani, S.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. The effect of oral curcumin supplementation on health-related quality of life: A systematic review and meta-analysis of randomized controlled trials. J. Affect. Disord 2021, 278, 627–636.
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: A randomized double-blind placebo-controlled trial. Phytother. Res. 2014, 28, 1461–1467.
- Waseem, M.; Parvez, S.; Tabassum, H. Mitochondria as the Target for the Modulatory Effect of Curcumin in Oxaliplatin-induced Toxicity in Isolated Rat Liver Mitochondria. Arch. Med. Res. 2017, 48, 55–63.
- Ruiz de Porras, V.; Layos, L.; Martinez-Balibrea, E. Curcumin: A therapeutic strategy for colorectal cancer? Semin. Cancer Biol. 2021, 73, 321–330.
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86.
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376.
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive Effect of Curcumin Against Chemotherapy-Induced Side-Effects. Front. Pharm. 2018, 9, 1374.
- Lu, Y.; Wu, S.; Xiang, B.; Li, L.; Lin, Y. Curcumin Attenuates Oxaliplatin-Induced Liver Injury and Oxidative Stress by Activating the Nrf2 Pathway. Drug Des. Devel. 2020, 14, 73–85.
- Al Moundhri, M.S.; Al-Salam, S.; Al Mahrouqee, A.; Beegam, S.; Ali, B.H. The effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: Some behavioral, biochemical, and histopathological studies. J. Med. Toxicol. 2013, 9, 25–33.
- Waseem, M.; Parvez, S. Neuroprotective activities of curcumin and quercetin with potential relevance to mitochondrial dysfunction induced by oxaliplatin. Protoplasma 2016, 253, 417–430.
- Sun, C.Y.; Zhang, Q.Y.; Zheng, G.J.; Feng, B. Phytochemicals: Current strategy to sensitize cancer cells to cisplatin. Biomed. Pharm. 2019, 110, 518–527.
- Abadi, A.J.; Mirzaei, S.; Mahabady, M.K.; Hashemi, F.; Zabolian, A.; Hashemi, F.; Raee, P.; Aghamiri, S.; Ashrafizadeh, M.; Aref, A.R.; et al. Curcumin and its derivatives in cancer therapy: Potentiating antitumor activity of cisplatin and reducing side effects. Phytother. Res. 2022, 36, 189–213.
- Hussain, Y.; Islam, L.; Khan, H.; Filosa, R.; Aschner, M.; Javed, S. Curcumin-cisplatin chemotherapy: A novel strategy in promoting chemotherapy efficacy and reducing side effects. Phytother. Res. 2021, 35, 6514–6529.
- Fetoni, A.R.; Eramo, S.L.; Paciello, F.; Rolesi, R.; Podda, M.V.; Troiani, D.; Paludetti, G. Curcuma longa (curcumin) decreases in vivo cisplatin-induced ototoxicity through heme oxygenase-1 induction. Otol. Neurotol. 2014, 35, e169-77.
- Mendonca, L.M.; da Silva Machado, C.; Teixeira, C.C.; de Freitas, L.A.; Bianchi Mde, L.; Antunes, L.M. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology 2013, 34, 205–211.
- Ouyang, M.; Luo, Z.; Zhang, W.; Zhu, D.; Lu, Y.; Wu, J.; Yao, X. Protective effect of curcumin against irinotecaninduced intestinal mucosal injury via attenuation of NFkappaB activation, oxidative stress and endoplasmic reticulum stress. Int. J. Oncol. 2019, 54, 1376–1386.
- Guclu, O.; Doganlar, O.; Yuksel, V.; Doganlar, Z.B. FOLFIRI-Mediated Toxicity in Human Aortic Smooth Muscle Cells and Possible Amelioration with Curcumin and Quercetin. Cardiovasc. Toxicol. 2020, 20, 139–154.
- Scontre, V.A.; Martins, J.C.; de Melo Sette, C.V.; Mutti, H.; Cubero, D.; Fonseca, F.; Del Giglio, A. Curcuma longa (Turmeric) for Prevention of Capecitabine-Induced Hand-Foot Syndrome: A Pilot Study. J. Diet. Suppl. 2018, 15, 606–612.
- Sabet, N.S.; Atashbar, S.; Khanlou, E.M.; Kahrizi, F.; Salimi, A. Curcumin attenuates bevacizumab-induced toxicity via suppressing oxidative stress and preventing mitochondrial dysfunction in heart mitochondria. Naunyn Schmiedebergs Arch. Pharm. 2020, 393, 1447–1457.
- Belcaro, G.; Hosoi, M.; Pellegrini, L.; Appendino, G.; Ippolito, E.; Ricci, A.; Ledda, A.; Dugall, M.; Cesarone, M.R.; Maione, C.; et al. A controlled study of a lecithinized delivery system of curcumin (Meriva(R)) to alleviate the adverse effects of cancer treatment. Phytother. Res. 2014, 28, 444–450.
- Kalluru, H.; Kondaveeti, S.S.; Telapolu, S.; Kalachaveedu, M. Turmeric supplementation improves the quality of life and hematological parameters in breast cancer patients on paclitaxel chemotherapy: A case series. Complement. Clin. Pract. 2020, 41, 101247.
- Martinez-Balibrea, E.; Martinez-Cardus, A.; Gines, A.; Ruiz de Porras, V.; Moutinho, C.; Layos, L.; Manzano, J.L.; Buges, C.; Bystrup, S.; Esteller, M.; et al. Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol. Cancer 2015, 14, 1767–1776.
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309.
- Ruiz de Porras, V.; Bystrup, S.; Martinez-Cardus, A.; Pluvinet, R.; Sumoy, L.; Howells, L.; James, M.I.; Iwuji, C.; Manzano, J.L.; Layos, L.; et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-kappaB signalling pathway. Sci. Rep. 2016, 6, 24675.
- Wang, S.; Liu, Z.; Wang, L.; Zhang, X. NF-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol. Immunol. 2009, 6, 327–334.
- Patel, M.; Horgan, P.G.; McMillan, D.C.; Edwards, J. NF-kappaB pathways in the development and progression of colorectal cancer. Transl. Res. 2018, 197, 43–56.
- Jobin, C.; Bradham, C.A.; Russo, M.P.; Juma, B.; Narula, A.S.; Brenner, D.A.; Sartor, R.B. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J. Immunol. 1999, 163, 3474–3483.
- Rajitha, B.; Belalcazar, A.; Nagaraju, G.P.; Shaib, W.L.; Snyder, J.P.; Shoji, M.; Pattnaik, S.; Alam, A.; El-Rayes, B.F. Inhibition of NF-kappaB translocation by curcumin analogs induces G0/G1 arrest and downregulates thymidylate synthase in colorectal cancer. Cancer Lett. 2016, 373, 227–233.
- Zhang, N.; Hao, Y.; Liu, H.; Yu, Q.; Bo, B.; Liang, J. Combined anti-cancer effects of curcumin and oxaliplatin on colon carcinoma colo205 cells using transplanted nude mice. Pak. J. Pharm. Sci. 2021, 34, 2021–2025.
- Howells, L.M.; Sale, S.; Sriramareddy, S.N.; Irving, G.R.; Jones, D.J.; Ottley, C.J.; Pearson, D.G.; Mann, C.D.; Manson, M.M.; Berry, D.P.; et al. Curcumin ameliorates oxaliplatin-induced chemoresistance in HCT116 colorectal cancer cells in vitro and in vivo. Int. J. Cancer 2011, 129, 476–486.
- Ozawa-Umeta, H.; Kishimoto, A.; Imaizumi, A.; Hashimoto, T.; Asakura, T.; Kakeya, H.; Kanai, M. Curcumin beta-D-glucuronide exhibits anti-tumor effects on oxaliplatin-resistant colon cancer with less toxicity in vivo. Cancer Sci. 2020, 111, 1785–1793.
- Shakibaei, M.; Mobasheri, A.; Lueders, C.; Busch, F.; Shayan, P.; Goel, A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-kappaB and Src protein kinase signaling pathways. PLoS ONE 2013, 8, e57218.
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250.
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Harikumar, K.B.; Deorukhkar, A.; Gelovani, J.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer 2009, 125, 2187–2197.
- Patel, B.B.; Gupta, D.; Elliott, A.A.; Sengupta, V.; Yu, Y.; Majumdar, A.P. Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res. 2010, 30, 319–325.
- Patel, B.B.; Sengupta, R.; Qazi, S.; Vachhani, H.; Yu, Y.; Rishi, A.K.; Majumdar, A.P. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int. J. Cancer 2008, 122, 267–273.
- Kanwar, S.S.; Yu, Y.; Nautiyal, J.; Patel, B.B.; Padhye, S.; Sarkar, F.H.; Majumdar, A.P. Difluorinated-curcumin (CDF): A novel curcumin analog is a potent inhibitor of colon cancer stem-like cells. Pharm. Res. 2011, 28, 827–838.
- Codony-Servat, J.; Cuatrecasas, M.; Asensio, E.; Montironi, C.; Martinez-Cardus, A.; Marin-Aguilera, M.; Horndler, C.; Martinez-Balibrea, E.; Rubini, M.; Jares, P.; et al. Nuclear IGF-1R predicts chemotherapy and targeted therapy resistance in metastatic colorectal cancer. Br. J. Cancer 2017, 117, 1777–1786.
- Chen, X.; Yeung, T.K.; Wang, Z. Enhanced drug resistance in cells coexpressing ErbB2 with EGF receptor or ErbB3. Biochem. Biophys. Res. Commun. 2000, 277, 757–763.
- Watson, R.G.; Muhale, F.; Thorne, L.B.; Yu, J.; O’Neil, B.H.; Hoskins, J.M.; Meyers, M.O.; Deal, A.M.; Ibrahim, J.G.; Hudson, M.L.; et al. Amplification of thymidylate synthetase in metastatic colorectal cancer patients pretreated with 5-fluorouracil-based chemotherapy. Eur. J. Cancer 2010, 46, 3358–3364.
- Bohanes, P.; Labonte, M.J.; Lenz, H.J. A review of excision repair cross-complementation group 1 in colorectal cancer. Clin. Color. Cancer 2011, 10, 157–164.
- Han, W.; Yin, H.; Ma, H.; Wang, Y.; Kong, D.; Fan, Z. Curcumin Regulates ERCC1 Expression and Enhances Oxaliplatin Sensitivity in Resistant Colorectal Cancer Cells through Its Effects on miR-409–3p. Evid. Based Complement Altern. Med. 2020, 2020, 8394574.
- Agarwal, A.; Kasinathan, A.; Ganesan, R.; Balasubramanian, A.; Bhaskaran, J.; Suresh, S.; Srinivasan, R.; Aravind, K.B.; Sivalingam, N. Curcumin induces apoptosis and cell cycle arrest via the activation of reactive oxygen species-independent mitochondrial apoptotic pathway in Smad4 and p53 mutated colon adenocarcinoma HT29 cells. Nutr. Res. 2018, 51, 67–81.
- Li, G.; Fang, S.; Shao, X.; Li, Y.; Tong, Q.; Kong, B.; Chen, L.; Wang, Y.; Yang, J.; Yu, H.; et al. Curcumin Reverses NNMT-Induced 5-Fluorouracil Resistance via Increasing ROS and Cell Cycle Arrest in Colorectal Cancer Cells. Biomolecules 2021, 11, 1295.
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629.
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374.
- Cao, H.; Xu, E.; Liu, H.; Wan, L.; Lai, M. Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review. Pathol. Res. Pract. 2015, 211, 557–569.
- Yang, Y.; Wang, G.; Zhu, D.; Huang, Y.; Luo, Y.; Su, P.; Chen, X.; Wang, Q. Epithelial-mesenchymal transition and cancer stem cell-like phenotype induced by Twist1 contribute to acquired resistance to irinotecan in colon cancer. Int. J. Oncol. 2017, 51, 515–524.
- Yang, A.D.; Fan, F.; Camp, E.R.; van Buren, G.; Liu, W.; Somcio, R.; Gray, M.J.; Cheng, H.; Hoff, P.M.; Ellis, L.M. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin. Cancer Res. 2006, 12 Pt 1, 4147–4153.
- Zhang, C.; Xu, Y.; Wang, H.; Li, G.; Yan, H.; Fei, Z.; Xu, Y.; Li, W. Curcumin reverses irinotecan resistance in colon cancer cell by regulation of epithelial-mesenchymal transition. Anticancer Drugs 2018, 29, 334–340.
- Yin, J.; Wang, L.; Wang, Y.; Shen, H.; Wang, X.; Wu, L. Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF-beta/Smad2/3 signaling pathway. Onco. Targets 2019, 12, 3893–3903.
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367.
- Lu, Y.; Zhang, R.; Zhang, X.; Zhang, B.; Yao, Q. Curcumin may reverse 5-fluorouracil resistance on colonic cancer cells by regulating TET1-NKD-Wnt signal pathway to inhibit the EMT progress. Biomed. Pharm. 2020, 129, 110381.
- Najafi, M.; Mortezaee, K.; Majidpoor, J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019, 234, 116781.
- Yu, Y.; Kanwar, S.S.; Patel, B.B.; Nautiyal, J.; Sarkar, F.H.; Majumdar, A.P. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl. Oncol. 2009, 2, 321–328.
- Shakibaei, M.; Buhrmann, C.; Kraehe, P.; Shayan, P.; Lueders, C.; Goel, A. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS ONE 2014, 9, e85397.
- Su, P.; Yang, Y.; Wang, G.; Chen, X.; Ju, Y. Curcumin attenuates resistance to irinotecan via induction of apoptosis of cancer stem cells in chemoresistant colon cancer cells. Int. J. Oncol. 2018, 53, 1343–1353.
- Yu, Y.; Sarkar, F.H.; Majumdar, A.P. Down-regulation of miR-21 Induces Differentiation of Chemoresistant Colon Cancer Cells and Enhances Susceptibility to Therapeutic Regimens. Transl. Oncol. 2013, 6, 180–186.
- Elbadawy, M.; Hayashi, K.; Ayame, H.; Ishihara, Y.; Abugomaa, A.; Shibutani, M.; Hayashi, S.M.; Hazama, S.; Takenouchi, H.; Nakajima, M.; et al. Anti-cancer activity of amorphous curcumin preparation in patient-derived colorectal cancer organoids. Biomed. Pharm. 2021, 142, 112043.
- Zheng, X.; Yang, X.; Lin, J.; Song, F.; Shao, Y. Low curcumin concentration enhances the anticancer effect of 5-fluorouracil against colorectal cancer. Phytomedicine 2021, 85, 153547.
- He, W.T.; Zhu, Y.H.; Zhang, T.; Abulimiti, P.; Zeng, F.Y.; Zhang, L.P.; Luo, L.J.; Xie, X.M.; Zhang, H.L. Curcumin Reverses 5-Fluorouracil Resistance by Promoting Human Colon Cancer HCT-8/5-FU Cell Apoptosis and Down-regulating Heat Shock Protein 27 and P-Glycoprotein. Chin. J. Integr. Med. 2019, 25, 416–424.
- Hoffmann, U.; Kroemer, H.K. The ABC transporters MDR1 and MRP2: Multiple functions in disposition of xenobiotics and drug resistance. Drug Metab. Rev. 2004, 36, 669–701.
- Smith, A.J.; van Helvoort, A.; van Meer, G.; Szabo, K.; Welker, E.; Szakacs, G.; Varadi, A.; Sarkadi, B.; Borst, P. MDR3 P-glycoprotein, a phosphatidylcholine translocase, transports several cytotoxic drugs and directly interacts with drugs as judged by interference with nucleotide trapping. J. Biol. Chem. 2000, 275, 23530–23539.
- Germann, U.A. P-glycoprotein—A mediator of multidrug resistance in tumour cells. Eur. J. Cancer 1996, 32A, 927–944.
- Ruetz, S.; Gros, P. A mechanism for P-glycoprotein action in multidrug resistance: Are we there yet? Trends Pharm. Sci. 1994, 15, 260–263.
- Borst, P. Looking back at multidrug resistance (MDR) research and ten mistakes to be avoided when writing about ABC transporters in MDR. FEBS Lett. 2020, 594, 4001–4011.
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464.
- Montazami, N.; Kheir Andish, M.; Majidi, J.; Yousefi, M.; Yousefi, B.; Mohamadnejad, L.; Shanebandi, D.; Estiar, M.A.; Khaze, V.; Mansoori, B.; et al. siRNA-mediated silencing of MDR1 reverses the resistance to oxaliplatin in SW480/OxR colon cancer cells. Cell Mol. Biol. 2015, 61, 98–103.
- Beretta, G.L.; Benedetti, V.; Cossa, G.; Assaraf, Y.G.; Bram, E.; Gatti, L.; Corna, E.; Carenini, N.; Colangelo, D.; Howell, S.B.; et al. Increased levels and defective glycosylation of MRPs in ovarian carcinoma cells resistant to oxaliplatin. Biochem. Pharm. 2010, 79, 1108–1117.
- Ekblad, L.; Kjellstrom, J.; Johnsson, A. Reduced drug accumulation is more important in acquired resistance against oxaliplatin than against cisplatin in isogenic colon cancer cells. Anticancer Drugs 2010, 21, 523–531.
- Lee, J.H.; Um, J.W.; Lee, J.H.; Kim, S.H.; Lee, E.S.; Kim, Y.S. Can immunohistochemistry of multidrug-resistant proteins replace the histoculture drug response assay in colorectal adenocarcinomas? Hepatogastroenterology 2012, 59, 1075–1078.
- Marin, J.J.G.; Macias, R.I.R.; Monte, M.J.; Herraez, E.; Peleteiro-Vigil, A.; Blas, B.S.; Sanchon-Sanchez, P.; Temprano, A.G.; Espinosa-Escudero, R.A.; Lozano, E.; et al. Cellular Mechanisms Accounting for the Refractoriness of Colorectal Carcinoma to Pharmacological Treatment. Cancers 2020, 12, 2605.
- Cao, D.; Qin, S.; Mu, Y.; Zhong, M. The role of MRP1 in the multidrug resistance of colorectal cancer. Oncol. Lett. 2017, 13, 2471–2476.
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomark. Prev. 2005, 14, 120–125.
- Irving, G.R.; Howells, L.M.; Sale, S.; Kralj-Hans, I.; Atkin, W.S.; Clark, S.K.; Britton, R.G.; Jones, D.J.; Scott, E.N.; Berry, D.P.; et al. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration--a clinical pilot study including assessment of patient acceptability. Cancer Prev. Res. 2013, 6, 119–128.
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854.
- James, M.I.; Iwuji, C.; Irving, G.; Karmokar, A.; Higgins, J.A.; Griffin-Teal, N.; Thomas, A.; Greaves, P.; Cai, H.; Patel, S.R.; et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015, 364, 135–141.
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139.
This entry is offline, you can click here to edit this entry!
