Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Biosynthesis of gold nanoparticles (AuNPs) has been widely studied and described. In the age of bacterial drug resistance, an intensive search for new agents with antibacterial properties or a new form of antibiotics with effective action is necessary.
- biosynthesis
- gold nanoparticles
- plant extract
1. Introduction
Nanoparticles (NPs) can be obtained by various methods, and the increased interest in metallic NPs (MNPs) has forced the development of new synthesis strategies that are inexpensive, easy to carry out, and most importantly, environmentally friendly [1]. Recently, there has been increased interest in biological methods of obtaining MNPs. Unlike chemical syntheses, biological methods do not use toxic reducing, blocking and stabilizing compounds, which makes biosynthesis eco-friendly [2]. Moreover, the obtained NPs are biocompatible. On the other hand, physical methods require specialized equipment and large amounts of energy to carry out the synthesis process (e.g., generation of high pressure and temperature, ultrasound waves, UV radiation, etc.), which makes the synthesis process time-consuming and expensive [3].
MNPs, including gold NPs (AuNPs), are of great interest due to their intrinsic surface plasmon resonance (SPR) property. The SPR of AuNPs promotes their use in imaging diagnostics, anti-cancer therapy to induce local hypothermia, or as biosensors and biomarkers [4][5]. Additionally, AuNPs are highly valued for their unique biological properties such as biocompatibility, facile surface functionalization, catalytic activity and the ability to reveal cytotoxic and/or antimicrobial activity [5]. AuNPs having various types of envelopes, i.e., surfaces functionalized with active biomolecules which give them unique properties, are especially useful for antimicrobial applications [6][7][8]. Currently, the most widespread searches regarding AuNPs are those for specific biological properties. Biosynthesized AuNPs, which have specific biological properties due to the natural envelope formed during the biosynthesis process, are included in these searches [9]. Products of natural origin are used for the biosynthesis of AuNPs; most often, microorganisms and plants are used [3]. Biological methods for the synthesis of AuNPs are more attractive than conventional methods due to the greater availability and variety of the material used. In addition, the waste generated during ingredient preparation and post-reaction does not have a negative impact on the environment, and it is easier and cheaper to dispose of biosynthesis waste compared to the waste generated using conventional methods [3][10][11]. Recently, great progress has been made regarding the biological activity and the possible applications of AuNPs synthesized using plant extracts or substances isolated from them [10][12][13]. Due to this trend, the number of publications related to the synthesis of AuNPs from plant material has increased. The biological activity of the plant extracts themselves and the compounds they contain, as well as the vast variety of research material, have led to the increased acceptance of biosynthesis as a promising method to obtain AuNPs [4][7][13].
Plants have the ability to synthesize NPs both intracellularly and extracellularly. Intracellular methods of synthesizing NPs include culturing plants in metal-rich organic environments, e.g., metal-rich soil or hydroponic solutions. This method of obtaining NPs is usually aimed at applications outside of the biomedical space. In contrast, extracellular methods include the NPs synthesized using an extract obtained from the leaves, flowers, fruits or other parts of the selected plant [14]. Interestingly, extracts from different parts of the same plant can significantly differ in their composition and biological properties. Bioactive compounds present in plant extracts are primarily flavonoids, phenols, citric acid, ascorbic acid, polyphenols, terpenoids and alkaloids [9][12][14]. Many substances belonging to these groups of compounds have antioxidant properties and the ability to reduce gold ions to metallic gold.
The biosynthesis of AuNPs from plant extracts is an easy process. The selection of the plant from which the extract will be prepared is important because the plant species and the part from which the extract will be obtained affect the amount of reducing compounds and the formation of the envelope coating around the AuNPs (Figure 1) [1][15][16].
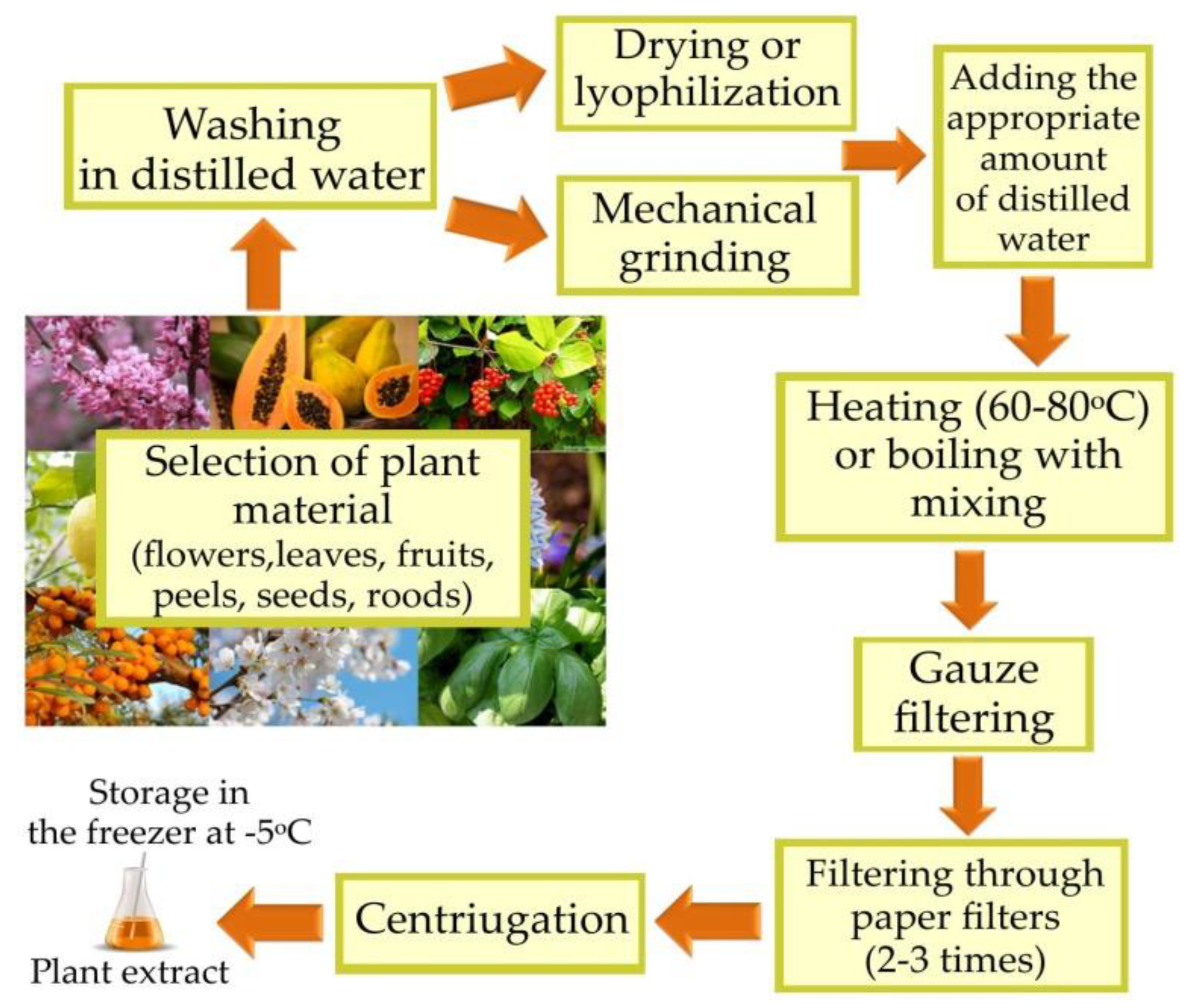
Figure 1. Preparation of plant extract.
First, the harvested plant material is thoroughly rinsed in distilled water. The next step is mechanically grinding the material with the addition of distilled water. Then, the mixture is heated to 60–80 °C or, in some cases, it is brought to the boil. Afterward, the obtained mixture is filtered several times. Finally, the filtrate is centrifuged [9]. The aqueous plant extract prepared in this way can be used for the biosynthesis of AuNPs or stored in a refrigerator (at −5 °C) until needed. The second reactant of the reaction is an aqueous solution of chloroauric acid (HAuCl4), which should be added in an appropriate amount to the prepared plant extract. The most commonly used concentrations of HAuCl4 solution range from 0.5–30 mM. The biosynthesis process is carried out in the dark at an appropriate temperature ranging from 25–90 °C [7][11]. The successful synthesis of AuNPs is evidenced by the color change of the mixture from yellow to pink, dark red or purple [17]. Particular attention is paid to the influence of physicochemical factors, i.e., concentration of reaction substrates, pH, temperature and duration of reaction, on the resultant NPs of the biosynthesis process. By selecting the appropriate synthesis conditions and the appropriate plant extract, the size and shape as well as the rate of formation and stability of AuNPs can be controlled [18]. Moreover, biosynthesized AuNPs can be complexed with other nanostructures or functionalized in a way to enhance their properties and increase their scope of application [19][20].
AuNPs are biocompatible, so they can easily bind to proteins or nucleic acids [21]. Biofunctionalized AuNPs with biologically active molecules incorporated within the NP envelope have recently become a popular subject of research due to their specific properties and their potential application in many areas, including as antibacterial agents [22]. Developing the most effective methods of fighting pathogens and preventing and treating bacterial diseases is a primary goal of current research [23]. The need for new antibacterial agents is due in part to the increasing resistance of bacteria to known antibiotics, but also due to the desire to increase the drug potency while reducing side effects [24]. Therefore, scientists aim to develop a broad acting antimicrobial agent which will reduce side effects to a minimum, and at the same time will not be toxic to bacteria that do not cause diseases but form bacterial flora of humans, such as Lactobacillus [23]. Notably, biosynthesized AuNPs would be advantageous as a new antibacterial agent because synthesized bacteria are unable to acquire resistance to them [25].
Studying the antibacterial activity of biosynthesized AuNPs is relatively complicated due to the multitude of parameters on which it depends, including the physicochemical conditions of the synthesis reaction (on which the shape and size of the AuNPs depend), the composition of the AuNPs’ envelope (on which the surface charge and stability of the AuNPs depend), the specificity of the interaction with bacterial cells at the molecular level, and parameters common to all pharmaceuticals (i.e., the type of bacteria and the concentration of AuNPs) [26][27]. Two terms are used to define the antimicrobial properties of tested substances: bacteriostatic and bactericidal. Bacteriostatic agents delay the growth of bacteria and stop their initial growth phase for a long time. Bactericidal agents completely inhibit bacterial growth [28][29]. The methods used to determine the antibacterial activity of other agents are also used to evaluate the antimicrobial activity of biosynthesized AuNPs. The most common method is the disk-diffusion method (zone of inhibition) and determining of the minimum inhibitory concentration (MIC50%) value, which is the minimum amount of a substance that inhibits the growth of 50% of bacteria, using the dilution method. The antibacterial activity of biosynthesized AuNPs differs depending on the type of bacteria, which is mainly related to the difference in the structure of the cell walls of Gram-positive and Gram-negative bacteria. Gram-negative bacteria have a more complex cell wall in terms of structure, but it is much thinner (from 2 to 10 nm) and much more susceptible to damage compared to the cell walls of Gram-positive bacteria [30]. The cell walls of Gram-negative bacteria contain one layer of murein (peptidoglycan) between two lipid membranes. The outer membrane consists of phospholipids, proteins and lipopolysaccharides [31]. The cell wall thickness of Gram-positive bacteria varies from 15 to 80 nm. The cell walls of Gram-positive bacteria are devoid of the outer lipid membrane, and the densely cross-linked murein contains teichoic acids, proteins and lipids [30][31].
Bacteria have developed mechanisms for protection and acquiring resistance. Therefore, it is important to learn about the specificity of the interaction of biosynthesized AuNPs with bacterial cells, determine the parameters on which the antibacterial activity of AuNPs depends, and identify what the mechanism of action of AuNPs on bacteria is and the path of cell death. The presented review aims to discuss the current knowledge of AuNPs synthesized from plant extracts for use as antibacterial agents while focusing on the suitability of plant products for the biosynthesis of AuNPs, the antibacterial properties of the biosynthesized AuNPs and plant extracts, and the parameters on which the antibacterial activity of biosynthesized AuNPs depends. Analyzing these research results will lead to conclusions regarding how biosynthesized AuNPs interact with bacterial cells and the potential mechanisms of their antibacterial action.
2. Biosynthesis of AuNPs Using Plants
2.1. Reduction Potential of Plant Extracts
Plants are a source of biologically active compounds that not only possess antioxidant properties, but also act as reducing agents in the biosynthesis reaction [8][11][12][32]. The total reduction capacity of plant extracts can be determined by studying electron transfer with antioxidants by reaction with the Folin–Ciocalteu reagent, the DPPH radical, or using one of the electrochemical methods [33][34]. The reduction potential of plant extracts may vary significantly depending on the composition of bioactive compounds [1][3][35]. Well-known reducing substances include secondary metabolites of plants such as sugars, terpenoids, polyphenols, alkaloids and proteins, most of which possess antioxidant properties [1][15][36] (Table 1). The in vitro antioxidant activity and reduction potential of Crassocephalum rubens leaf extract were investigated, and the obtained AuNPs synthesized using this extract were deemed suitable for future applications. The antioxidant potential of the post-reaction mixture was lower than that of the extract itself. Thus, the obtained results indicate that substances with antioxidant properties are associated with the reduction of gold ions to metallic gold [37]. The most common antioxidants in plant extracts are terpenoids and polyphenols. Terpenoids are a group of organic polymers that consist of five-carbon isoprene units. In contrast, flavonoids are a large group of polyphenolic compounds that includes anthocyanins, isoflavonoids, flavonols, chalcones, flavones, and flavanones [38][39]. Terpenoids and flavonoids can actively reduce metal ions to NPs because they contain various functional groups [40][41]. Additionally, monosaccharides can play a reducing role. Monosaccharides that contain a ketone group can act as an antioxidant only when the ketone is converted tautomerically into an aldehyde [40][41][42]. Moreover, the reducing capacity of disaccharides and polysaccharides depends on the composition of the monosaccharides and their ability to share an aldehyde group with the metal ion. Amino acids such as lysine, cysteine, tryptophan, tyrosine, arginine and methionine possess a high ability to bind various metal ions and reduce them [40][43].
Thus, the process of AuNP synthesis from plant extracts is closely related to the reduction potential and the presence of appropriate functional groups [44]. Three factors have a clear impact on biosynthesis efficiency. These include: the degree of reduction by metal ions (reduced by individual substances contained in the extract), the concentration of the reducing compounds and the composition of bioactive compounds forming the envelope stabilizing the AuNP [18]. Greater total content of reducing substances in the plant extract accelerates AuNP formation, increases the fraction of small NPs and increases the stability of the AuNPs [12][18][45]. The efficiency of the MNP biosynthesis process also depends on the electrochemical potential of a given metal ion [40]. The reduction potential of all noble metal salts ranges from 0.35 to 1.0 V. Any type of metal ions can be reduced to MNPs, provided that the reduction potential of the extract is greater than +0.16 V [44]. Hossanisaadi et al. (2021) screened and compared studies of plant extracts in terms of their ability to reduce gold ions. The review presents the results regarding the ability of the extracts from 27 plants, including Rosa damascena, Juglans regia, Caccinia macranthera, Urtica dioica, Areca catechu and Anethum graveolens, which are used in traditional medicine in the Middle East, to reduce gold ions. Extracts were prepared from various parts of the plants. Additionally, 28 new plants with suitable extracts were also identified. The derived extracts were able to successfully reduce gold ions during biosynthesis [46].
Plant extracts, especially fruit extracts, contain high concentrations of reducing compounds. For example, blackberries, blueberries, grapes, Citrullus lanatus, Cornus mas, Punica granatum, and Terminalia arjuna extracts contain large amounts of flavonoids, phenolic compounds, anthocyanins, saccharides, ascorbic acid and other vitamins [47][48][49][50][51]. Biosynthesis carried out using the extracts from these plants is more effective and less expensive compared to traditional chemical synthesis due to the abundance and presence of naturally occurring reducing agents [9]. In order to investigate the reducing properties of the Papaver somniferum extract, the synthesis of AuNPs was carried out. Moreover, they discovered that methanol extract has a high reducing potential with a high affinity for gold cations. The resulting spherical AuNPs were 77 nm in diameter and stabilized by phytochemicals present within the extract [52]. On the other hand, the synthesis of AuNPs carried out with the use of Artemisia capillaris extract showed that the composition of the plant extract had a significant impact on biosynthesis. The extract contained saponins, amino acids, phenolic compounds, flavonoids and diterpenes, but only flavonoids, phenolic compounds and amino acids were involved in the synthesis of AuNPs [53][54]. Examination of the composition of amino acids in the lyophilized extract of Galaxaura elongata revealed the presence of glutamic acid, asparagine, leucine, lysine, glycine and alanine. Amino acids were responsible for the reduction and stabilization of AuNPs, but sulphate polysaccharides and polypeptides also played a role [55]. Presumably, the reduction of gold ions by amino acids is due to the hydroxyl and carboxyl groups [56].
The influence of the extract composition, particularly the reducing compounds present in the extract, impact the size of AuNPs generated via biosynthesis. During the synthesis of AuNPs with ethanolic extract from black tea, the tannins acted as a reducing and stabilizing agent [57]. Spherical NPs were obtained with a bimodal size distribution of 10 nm and 3 nm for two fractions of AuNPs. Similarly, AuNPs synthesized using the Plumeria alba extract also resulted in a bimodal size distribution with spherical NPs either in the range of 20–30 nm or 80–150 nm [57]. Spherical AuNPs synthesized with fruit infusion from Medinilla speciosa were 200–450 nm in diameter and the phenolic compounds were responsible for the reduction of gold ions and stabilization of the AuNPs [36]. During the synthesis of AuNPs using the Mimosa tenuiflora extract, the v/v ratio of the reagents had a significant impact on the biological activity of the obtained AuNPs. However, neither the size of the AuNPs nor the composition of the plant extract were found to impact the biological activity of the resulting AuNPs [10].
Plants produce numerous secondary metabolites with antioxidant properties and enzymes that prevent oxidative damage to cell organelles and their contents. Flavonoids, flavonoid glycosides and vitamins, such as ascorbic acid isolated from plant extract, have also been shown to reduce gold ions [58]. Leaf extracts from medicinal plants are used for most of the AuNPs synthesis reactions. Active herbal compounds such as polyphenols are involved in the reduction of gold ions and the stabilization of AuNPs [54][59]. Active compounds isolated from the extract of Ocimum sanctum, such as apigenin, cirsimaritin, rosmarinic acid, estragole, linalool, carvacrol and urosolic acid, have numerous pharmaceutical applications, and the ligands of these compounds can reduce metal ions [60]. In the case of AuNPs synthesized using fruit extract from Genipa americana, substances such as genipin, genipaol, geniposide and ranolazine acted as reductants of gold ions. During AuNP synthesis using the extract of Lycopersicon esculentum, citric and ascorbic acids also had the ability to reduce gold ions [61][62].
AuNPs of various sizes were synthesized using pectins isolated from Musa paradisiaca fruit extracts and orange peels [63][64][65][66][67][68][69][70]. The resultant AuNPs were biocompatible with bacterial cells, cytotoxic against HeLa and HepG2 cell lines and zebrafish embryos, and showed anti-inflammatory activity [68][69][70]. Curcumin isolated from Curcuma longa was also investigated as a reducing and stabilizing agent for AuNPs. Curcumin is well known and primarily investigated due to its anti-cancer properties. Moreover, many research teams have successfully used curcumin to synthesize AuNPs under various pH and temperature conditions [71].
Table 1. Compounds responsible for creating the reducing potential of plant extracts.
| Compounds | Plant | Kind of Extract | References |
|---|---|---|---|
| Phenolic compounds, flavonoids | Crassocephalum rubens | Leaf; water extract |
[37] |
| Anthocyanins | Cornus mas | Fruit; water extract |
[47] |
| Anthocyanins | Punica granatum | Fruit; water extract |
[48] |
| Cholidonic, superbine, colchicine, gloriosol, phytosterils and stigmasterin | Gloriosa superba | Leaf; water extract |
[49] |
| Pectins, ribose, phenolic compounds | Papaver somniferum | Leaf; methanol extract |
[52] |
| Amino acids, phenolic compounds, flavonoids | Artemisia capillaris | Whole plant; water extract |
[53][54] |
| Glutanic acid, asparagine, leucine, lysine, glycine, alanine | Galaxaura elongata | Whole plant; water extract |
[55][56] |
| Tannins | Black tea | Leaf; ethanol extract |
[57] |
| Phenolic compounds | Medinilla speciosa | Fruit; water extract |
[36] |
| Catechins, ascorbic acid | Mimosa tenuiflora | Tree bark; water/ethanol extract |
[10] |
| Estragole, linalool, carvacral, urosalic acid, cirsimarin, rosmarinic acid | Ocinum sanctum | Flower and leaf; water extract |
[60] |
| Genipin, genipol, geniposide, ronolazine | Genipa americana | Fruit; water extract |
[61] |
| Citric and ascorbic acid | Lycopersicon esculentum | Fruit; water extract |
[62] |
| Pectins | Musa paradisiaca | Fruit; water extract |
[63][64][65][66][67][68][69][70] |
| Pectins | Citrus sinensis | Peels; water extract |
[63][64][65][66][67][68][69][70] |
| Curcumin | Curcuma longa | Water solution | [71] |
2.2. Mechanism of AuNPs Biosynthesis Using Plants
The biosynthesis of MNPs can take place through biogenesis and bioreduction. Biogenesis utilizes microorganisms. In contrast, only bioreduction is possible when using plant extracts or substances isolated from plants [72]. The process of AuNPs biosynthesis begins with the reduction of gold ions, i.e., activation, which depends on the reducing potential of the extract. The next stage of biosynthesis is the growth of the NPs [63][73]. This process involves increasing the size of the NP nuclei (seeds) and the merging of the NP nuclei into clusters. The last stage of the process, i.e., termination, continues until thermodynamic equilibrium is achieved and results in the formation of the final shape and size of the AuNPs (Figure 2) [18][73].
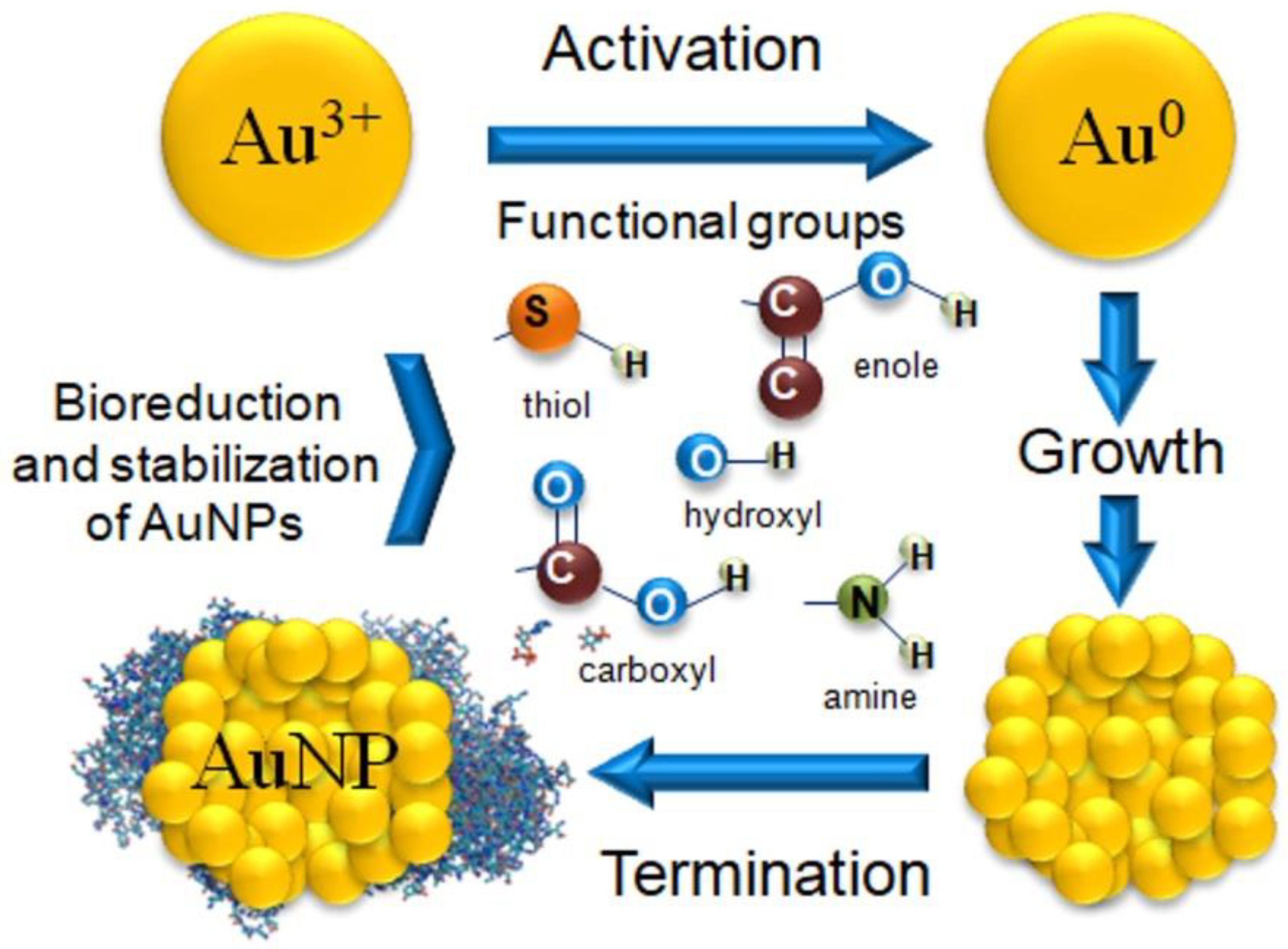
Figure 2. Mechanism of AuNPs biosynthesis and functional groups involved in the reduction of gold ions and stabilization of AuNPs.
The shape and size of AuNPs are influenced by electrostatic interactions between bioactive compounds derived from the plant extract and metallic gold [74]. The source of gold ions is the HAuCl4 solution in which three Cl atoms are covalently bonded and the fourth is coordinated. Many studies show that the mechanism of the reduction reaction depends on the number of Cl ligands in the metal complex, which is the source of the energy differences during the reaction [75]. For example, the synthesis of AuNPs was performed using 1,8-cineole obtained from the extract of Eucalyptus, an organic compound belonging to the terpenes. They discovered that the oxidation of 1,8-cineole initiated the entire biosynthesis process. Thus, the presence of a water molecule was necessary for energy reduction, and the bioreduction process itself took place in several stages [76].
The presence of hydroxyl or amino groups in the plant extract play an important role in the process of reducing gold ions to metallic gold. This process can take place during an oxidation reaction or due to the formation of specific quinine forms [43][60]. Gold reduction has also been demonstrated during the tautomeric conversion of flavonoids (from the enol form to the ketone form). In this reaction, a reactive hydrogen atom is released which can reduce gold ions to metallic gold [43]. The internal mechanism of the transformation of flavonoids from ketones to carboxylic acids may also be responsible for the reduction of gold ions [9]. In the case of AuNP synthesis using Garcinia cambogia and Pyrus fruit extracts, saccharides acted as a reducing agent. The reduction of gold ions most likely involves the oxidation of an aldehyde group to a carboxyl group by nucleophilic addition of a hydroxyl group [77][78]. Numerous plant extracts contain proteins with reducing potential. However, a protein’s ability to reduce gold ions varies based on its amino acid sequences [79].
2.3. Conditions of Biosynthesis Reaction
Physicochemical parameters have a significant impact on the course of each reaction, including biosynthesis, affecting the rate of the reaction and the size, shape and stability of the obtained AuNPs [18][35]. Moreover, the concentration of reactants, temperature, pH and the duration of the reaction have a decisive influence on the products of biosynthesis [80].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14122599
References
- Chahardoli, A.; Karimi, N.; Sadeghi, F.; Fattahi, A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 579–588.
- Mikhailova, E.O. Gold nanoparticles: Biosynthesis and potential of biomedical application. J. Func. Biomater. 2021, 12, 70.
- Singh, P.; Pandit, S.; Garnaes, J.; Tunjic, S.; Mokkapati, V.R.S.S.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591.
- Devasvaran, K.; Lim, V. Green synthesis of metallic nanoparticles using pectin as a reducing agent: A synthetic review of the biological activities. Pharma. Biol. 2021, 59, 491–503.
- Das, L.J.; Velusamy, P. Catalytic reduction of methylene blue using biegonic gold nanoparticles from Sesbania grandiflora. J. Inst. Chem. Eng. 2014, 45, 12285.
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556.
- Tian, E.-K.; Wang, Y.; Ren, R.; Zheng, W.; Liao, W. Gold nanoparticle: Recent progress on its antibacterial applications and mechanisms. J. Nanomater. 2021, 2021, 1–18.
- Mobed, A.; Hasanzadeh, M.; Seidi, F. Anti-bacterial activity of gold nanoparticles as a new nanomaterial weapon to combat phatogenic agents: Recent advances and challenges. RSC Adv. 2021, 11, 34688–34698.
- Timoszyk, A. A review of the biological synthesis of gold nanoparticles using fruit extracts: Scientific potential and application. Bull. Mater. Sci. 2018, 41, 154–165.
- Rodríguez-León, E.; Rodríguez-Vázquez, B.E.; Martínez-Higuera, A.; Rodríguez-Beas, C.; Larios- Rodríguez, E.; Navarro, R.E.; López-Esparza, R.; Iñiguez-Palomares, A. Synthesis of gold nanoparticles using Mimosa tenuiflora extract, assessments of cytotoxicity, cellular uptake, and catalysis. Nanoscale Res. Lett. 2019, 14, 334.
- Castro, L.; Blázquez, M.L.; Muńoz, J.A.; González, F.G.; Ballester, A. Mechanism and applications of metal nanoparticles prepared by bio-mediated process. Rev. Adv. Sci. Eng. 2014, 3, 199–216.
- Stozhko, N.Y.; Bukharinova, M.A.; Khamzina, E.I.; Tarasov, A.V.; Vidrevich, M.B.; Brainina, K.Z. The effect of the antioxidant activity of plant extracts on the properties of gold nanoparticles. Nanomaterials 2019, 9, 1655.
- Yazdani, E.; Talebi, M.; Zarshenas, M.M.; Moein, M. Evaluation of possible antioxidant activities of barberry solid formulation, a selected formulation from Traditional Persian Medicine (TPM) via various procedures. Biointer. Res. Appl. Chem. 2019, 9, 4517–4521.
- Sathishkumar, P.; Gu, F.L.; Zhan, Q.; Palvannan, T.; Yusof, A.R.M. Flavonoids mediated ‘green’ nanomaterials: A novel nanomedicine system to treat various diseases—Current trends and future perspective. Mater. Lett. 2018, 210, 26–30.
- Rao, Y.; Inwati, G.K.; Singh, M. Green synthesis of capped gold nanoparticles and their effect on Gram-positive and Gram-negative bacteria. Future Sci. OA 2017, 3, 239–254.
- Naeem, G.A.; Jaloot, A.S.; Owaid, M.N.; Muslim, R.F. Green synthesis of gold nanoparticles from Coprinus comatus, Agaricaceae, and the effect of ultraviolent irradiation on their characteristics. Walailak J. Sci. Technol. 2021, 18, 9396.
- Xu, X.Y.; Tran, T.H.M.; Perumalsamy, H.; Sanjeevram, D.; Kim, Y.-J. Biosynthetic gold nanoparticles of Hibiscus syriacus L. callus potentiates anti-inflammation efficacy via an autophagy-dependent mechanism. Mater. Sci. Eng. 2021, 124, 112035–112049.
- Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z.; Zeiri, Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 2014, 9, 4007–4021.
- Ahmad, F.; Ashraf, N.; Ashraf, T.; Zhou, R.-B.; Yin, D.-C. Biological synthesis of metallic nanoparticles (MNPs) by plants and microbes: Their cellular uptake, biocompatibility, and biomedical applications. Appl. Microbiol. Biotechnol. 2019, 103, 2913–2935.
- Kharissova, O.V.; Kharisov, B.I.; Oliva González, C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378.
- Ahn, S.; Singh, P.; Castro-Aceituno, V.; Yasmin Simu, S.; Kim, Y.; Mathiyalagan, R.; Yang, D. Gold nanoparticles synthesized using Panax ginseng leaves suppress inflammatory-mediators production via blockade of NF-kB activation in microphages. Artif. Cells Nanomed. Biotechnol. 2017, 45, 270–276.
- Vadlapudi, V.; Kaladhar, D.S.V.G.K. Review: Green synthesis of silver and gold nanoparticles. Middle-East. J. Sci. Res. 2014, 19, 834–842.
- Dahiya, D.; Nigam, P.S. The gut microbiota influenced by the intake of probiotics and functional foods with prebiotics can sustain wellness and alleviate certain ailments like gut-inflammation and colon-cancer. Microorganisms 2022, 10, 665.
- Gupta, A.; Landis, R.F.; Rotello, V.M. Nanoparticle-based antimicrobials: Surface functionality is critical. F1000 Res. 2016, 5, 364–374.
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278.
- Zhang, J.; Mou, L.; Jiang, X. Surface chemistry of gold nanoparticles for health applications. Chem. Sci. 2020, 11, 923.
- Princy, K.F.; Gopinath, A. Optimization of physicochemical parameters in the biofabrication of gold nanoparticles using marine macroalgae Padina tetrastromatica and its catalytic efficacy in the degradation of organic dyes. J. Nanostruc. Chem. 2018, 8, 333–342.
- Correa, M.G.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469.
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial activity and resistance: Influencing factors. Front. Pharmacol. 2017, 8, 364.
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414.
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta 2016, 1858, 980–987.
- Madkur, L.H. Ecofriendly green biosynthesized of metallic nanoparticles: Bio-reduction mechanism, characterization and pharmaceutical applications in biotechnology industry. Glob. Drug Ther. 2018, 3, 1–11.
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/Capacity measurement. 2. Hydrogen atom transfer (HAT)-based, mixed-mode (electron transfer (ET)/HAT), and lipid peroxidation assays. J. Agric. Food Chem. 2016, 64, 1028–1045.
- Pisoschi, A.M.; Cimpeanu, C.; Predoi, G. Electrochemical methods for total antioxidant capacity and its main contributors determination: A review. Open Chem. 2015, 13, 824–856.
- Patil, M.P.; Kim, G.-D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92.
- Prihapsara, F.; Artanti, A.N.; Ni’mah, L.F.U. Characterization and antimicrobial activity of gold nanoparticles fruit infusion of Medinilla speciosa. J. Phys. Conf. Ser. 2022, 2190, 1–7.
- Adewale, O.B.; Egbeyemi, K.A.; Onwuelu, J.O.; Potts-Johnson, S.S.; Anadozie, S.O.; Fadaka, A.O.; Osukoya, O.A.; Aluko, B.T.; Johnson, J.; Obafemi, T.O.; et al. Biological synthesis of gold and silver nanoparticles using leaf extracts of Crassocephalum rubens and their comparative in vitro antioxidant activities. Heliyon 2020, 6, e05501.
- Jagessar, R.C. Plant extracts based nanoparticles, a good perspective in the development of drugs in nanomedicine. Mod. Appro. Drug Des. 2020, 3, 000556.
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; UI Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172–187.
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. ‘Green’ nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44.
- Yasmin, A.; Ramesh, K.; Raje, S. Optimization and stabilization of gold nanoparticles by using herbal plant extract with microwave heating. Nano Converg. 2014, 1, 12. Available online: http://www.nanoconvergencejournal.com/content/1/1/12 (accessed on 11 April 2014).
- Cherkas, A.; Holota, S.; Mdzinarashvili, T.; Gabbianelli, R.; Zarkovic, N. Glucose as a major antioxidant: When, what for and why it fails? Antioxidants 2020, 9, 140.
- Ramrakhiani, L.; Ghosh, S. Metallic nanoparticle synthesized by biological route: Safer candidate for diverse applications. IET Nanobiotechnol. 2018, 12, 392–404.
- Siddiqi, K.S.; Husen, A. Engineered gold nanoparticles and plant adaptation potential. Nanoscale Res. Lett. 2016, 11, 400–410.
- Fanoro, O.T.; Oluwafemi, O.S. Bactericidal antibacterial mechanism of plant synthesized silver, gold and bimetallic nanoparticles. Pharmaceutics 2020, 12, 1044.
- Hassanisaadi, M.; Bonjar, G.H.S.; Rahdar, A.; Pandey, S.; Hosseinipour, A.; Abdolshahi, R. Environmentally safe biosynthesis of gold nanoparticles using plant water extracts. Nanomaterials 2021, 11, 2033.
- Olenic, L.; Chiorean, I. Synthesis, characterization and application of nanomaterials based on noble metallic nanoparticles and anthocyanins. Int. J. Latest Res. Sci. Technol. 2015, 4, 6–22.
- Nadagouda, M.N.; Iyanna, N.; Lalley, J.; Han, C.; Dionysiou, D.D.; Varma, R.S. Synthesis of silver and gold nanoparticles using antioxidants from blackberry, blueberry, pomegranate, and turmeric extracts. ACS Sustain. Chem. Eng. 2014, 2, 1717–1723.
- Gopinath, K.; Karthika, V.; Gowri, S.; Senthilkumar, V.; Kumaresan, S.; Arumugam, A. Antibacterial activity of ruthenium nanoparticles synthesized using Gloriosa superba L. leaf extract. J. Nanosruc. Chem. 2014, 4, 83–89.
- Lokina, S.; Narayanan, V. Antimicrobial and anticancer activity of gold nanoparticles synthesized from grapes fruit extract. Chem. Sci. Trans. 2013, 2, 105–110.
- Gan, P.P.; Ng, S.H.; Huang, Y.; Li, S.F.Y. Green synthesis of gold nanoparticles using palm oil mill effluent (POME): A low-cost and eco-friendly viable approach. Bioresour. Technol. 2012, 113, 132–135.
- Wali, M.; Sajjad, A.S.; Sumaira, S.; Muhammad, N.; Safia, H.; Muhammad, J. Green synthesis of gold nanoparticles and their characterizations using plant extract of Papaver somniferum. Nano Sci. Nano Technol. 2017, 11, 118–127. Available online: www.tsijournals.com (accessed on 7 September 2017).
- Lim, S.H.; Ahn, E.-Y.; Park, Y. Green synthesis and catalytic activity of gold nanoparticles synthesized by Artemisia capillaris water extract. Nanoscale Res. Lett. 2016, 11, 474.
- Santhosh, P.B.; Genova, J.; Chamati, H. Green synthesis of gold nanoparticles: An eco-friendly approach. Chemistry 2022, 4, 345–369.
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green biosynthesis of gold nanoparticle using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, 3029–3039.
- Vijayaraghavan, K.; Nalini, S.P.K. Biotemplates in the green synthesis of silver nanoparticles. Biotechnol. J. 2010, 5, 1098–1110.
- Teimuri-mofrad, R.; Hadi, R.; Tahmasebi, B.; Farhoudian, S.; Mehravar, M.; Nasiri, R. Green synthesis of gold nanoparticles using plant extract: Mini-review. Nanochem. Res. 2017, 2, 8–19.
- Aljabali, A.A.A.; Akkam, Y.; Zoubi, M.S.A.; Al-Batayneth, K.M.; Al-Trad, B.; Alrob, O.A.; Alkilany, A.M.; Benamara, M.; Evans, D.J. Synthesis of gold nanoparticles using leaf extract of Ziziphus zizyphus and their antimicrobial activity. Nanomaterials 2018, 8, 174.
- Boomi, P.; Ganesan, R.; Poorani, G.P.; Jegatheeswaran, S.; Balakumar, C.; Prabu, H.G.; Anand, K.; Prabhu, N.M.; Jeyakanthan, J.; Saravanan, M. Phyto-engineered gold nanoparticles (AuNPs) with potential antibacterial, antioxidant, and wound healing activities under in vitro and in vivo conditions. Int. J. Nanomed. 2020, 15, 7553–7568.
- Sengani, M.; Grumezescu, A.M.; Rajeswari, V.D. Recent trends and methodologies in gold nanoparticle synthesis—A prospective review on drug delivery aspect. Open Nano 2017, 2, 37–46.
- Kumar, B.; Smita, K.; Cumbal, L.; Camacho, J.; Hernández-Gallegos, E.; De Guadalupe Chávez-López, M.; Grijalva, M.; Andrade, K. One pot phytosynthesis of gold nanoparticles using Genipa americana fruit extract and its biological applications. Mater. Sci. Eng. C 2016, 62, 725–731.
- Barman, G.; Maiti, S.; Laha, J.K. Bio-fabrication of gold nanoparticles using aqueous extract of red romato and its use as a colorimetric sensor. Nanoscale Res. Lett. 2013, 8, 181–190. Available online: www.nanoscalereslett.com/content/8/1/181 (accessed on 19 April 2013).
- Ahmad, M.Z.; Abdel-Wahab, B.A.; Alam, A.; Zafar, S.; Ahmad, J.; Ahmad, F.J.; Midoux, P.; Pichon, C.; Akhter, S. Toxicity of inorganic nanoparticles used in targeted drug delivery and other biomedical application: An updated account on concern of biomedical nanotoxicology. J. Nanosci. Nanotechnol. 2016, 16, 7873–7897.
- Borker, S.; Patole, M.; Moghe, A.; Pokharkar, V. Engineering of pectin-reduced gold nanoparticles for targeted delivery of an antiviral drug to macrophages: In vitro and in vivo assessment. Gold Bull. 2017, 50, 235–246.
- Borker, S.; Pokharkar, V. Engineering of pectin-capped gold nanoparticles for delivery of doxorubicin to hepatocarcinoma cells: An insides into mechanism of cellular uptake. Artif. Cells Nanomed. Biotechnol. 2018, 46, 826–835.
- Chen, H.; Dorrigan, A.; Saad, S.; Hare, D.J.; Cortie, M.B.; Valenzuela, S.M. In vivo study of spherical gold nanoparticles: Inflammatory effects and distribution in mice. PLoS ONE 2013, 8, e58208.
- Nigoghossian, K.; dos Santos, M.V.; Barud, H.S.; da Silva, R.R.; Rocha, L.A.; Caiut, J.M.A.; de Assunção, R.M.N.; Spanhel, L.; Poulain, M.; Messaddeq, Y.; et al. Orange pectin mediated growth and stability of aqueous gold and silver nanocolloids. Appl. Surf. Sci. 2015, 341, 28–36.
- Suganya, K.U.; Govindaraju, K.; Kumar, V.G.; Karthick, V.; Parthasarathy, K. Pectin mediated gold nanoparticles induces apoptosis in mammary adenocarcinoma cell lines. Int. J. Biol. Macromol. 2016, 93, 1030–1040.
- Suganya, K.U.; Govindaraju, K.; Sivaraman, D.; Selvaraj, R.; Manikandan, R.; Kumar, V.G. Nanotoxicity assessment of functionalized gold nanoparticles in Sprague-Dawley rats. J. Clust. Sci. 2017, 28, 2933–2951.
- Tan, H.; Chen, W.; Liu, Q.; Yang, G.; Li, K. Pectin oligosaccharides ameliorate colon cancer by regulating oxidative stress- and inflammation-activated signalling pathways. Front. Immunol. 2018, 9, 1504.
- Patra, D.; Kurdi, R.E. Curcumin as a novel reducing and stabilizing agent for the green synthesis of metallic nanoparticles. Green Chem. Lett. Rev. 2021, 14, 474–487.
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639.
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991.
- Sanchez-Mendieta, V.; Vilchis-Nestor, A.R. Green Synthesis of Noble Metal (Au, Ag, Pt) Nanoparticles, Assisted by Plant-Extracts; Su, Y.-H., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 391–408.
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem. Rev. 2019, 119, 4819–4880.
- Singh, A.K.; Srivastava, O.N. One-step green synthesis of gold nanoparticles using black cardamom and effect of pH on its synthesis. Nanoscale Res. Lett. 2015, 10, 353.
- Rajan, A.; Vilas, V.; Philip, D. Studies on catalytic, antioxidant, antibacterial and anticancer activities of biogenic gold nanoparticles. J. Mol. Liq. 2015, 212, 331–339.
- Ghodake, G.S.; Deshpande, N.G.; Lee, Y.P.; Jin, E.S. Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf. B 2010, 75, 584–589.
- Amina, S.J.; Guo, B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int. J. Nanomed. 2020, 15, 9823–9857.
- Kumari, M.; Mishra, A.; Pandey, S.; Singh, S.P.; Chaudhry, V.; Mudiam, M.K.R.; Shukla, S.; Kakkar, P.; Nautiyal, C.S. Physico-chemical condition optimization during biosynthesis lead to development of improved and catalytically efficient gold nano particles. Sci. Rep. 2016, 6, 27575.
This entry is offline, you can click here to edit this entry!
