A clinical study carried out previously by our group has demonstrated that yogurt manufactured with a plant-derived lactic acid bacterium, Lactobacillus plantarum SN13T, significantly reduces the γ-glutamyl transpeptidase (γ-GTP) level as a liver-function parameter. We show that with the oral administration of live SN13T cells, alcohol-poisoning symptoms in mice are improved, and the condition does not become fatal. However, prior to the simultaneous administration with ethanol, when the cells were heat-killed or sonicated, the improvement was not observed, and almost all of the mice died. In addition, the dysbiosis of the intestinal microbiota observed in the mice administered with ethanol was restored by simultaneous administration with live SN13T cells. Furthermore, by analyzing the metabolites detected in contents from the mouse cecum, it was demonstrated that the increase in nonvolatile putrefactive amines observed in the ethanol-administration group was reduced by simultaneous administration with live SN13T cells. Judging from these results, the lactic acid bacterial cells capable of reaching the living bowels prevent ethanol-induced poisoning and restore the intestinal microbiota.
- alcohol-poisoning symptoms
- Lactobacillus plantarum
- live lactic acid bacteria
1. Introduction
Lactic acid bacteria (LAB) are recognized as “probiotics.” The word “probiotics” is defined as “the live microorganisms conferring a health benefit on the host when administered in adequate amounts”[1], and probiotic LAB strains traditionally have been used to manufacture fermented foods. It has been reported that some LAB cells and fermented foods containing the bacteria have potent health benefits, such as promoting intestinal homeostasis, possessing anti-allergic properties, and preventing and improving obesity[2][3][4][5][6][7][8][9].
We have found that some strains stored in our plant-derived LAB library may be useful for preventive medicine, such as immune modulation, reduction of obesity, and anti-allergy[10][11][12][13]. Interestingly, it has been demonstrated through the clinical study carried out by our group that the yogurt manufactured with Lactobacillus plantarum SN13T stored in the library reduces significantly the serum γ-glutamyl transpeptidase (γ-GTP) value[14].
During the animal study using mice fed with ethanol, it has been observed that when live SN13T cells were orally administered to mice fed with a diet containing ethanol, death caused by the intake of ethanol was completely avoided. The result demonstrates that recovery from alcohol-poisoning symptoms in mice was observed only with oral administration of the live cells.
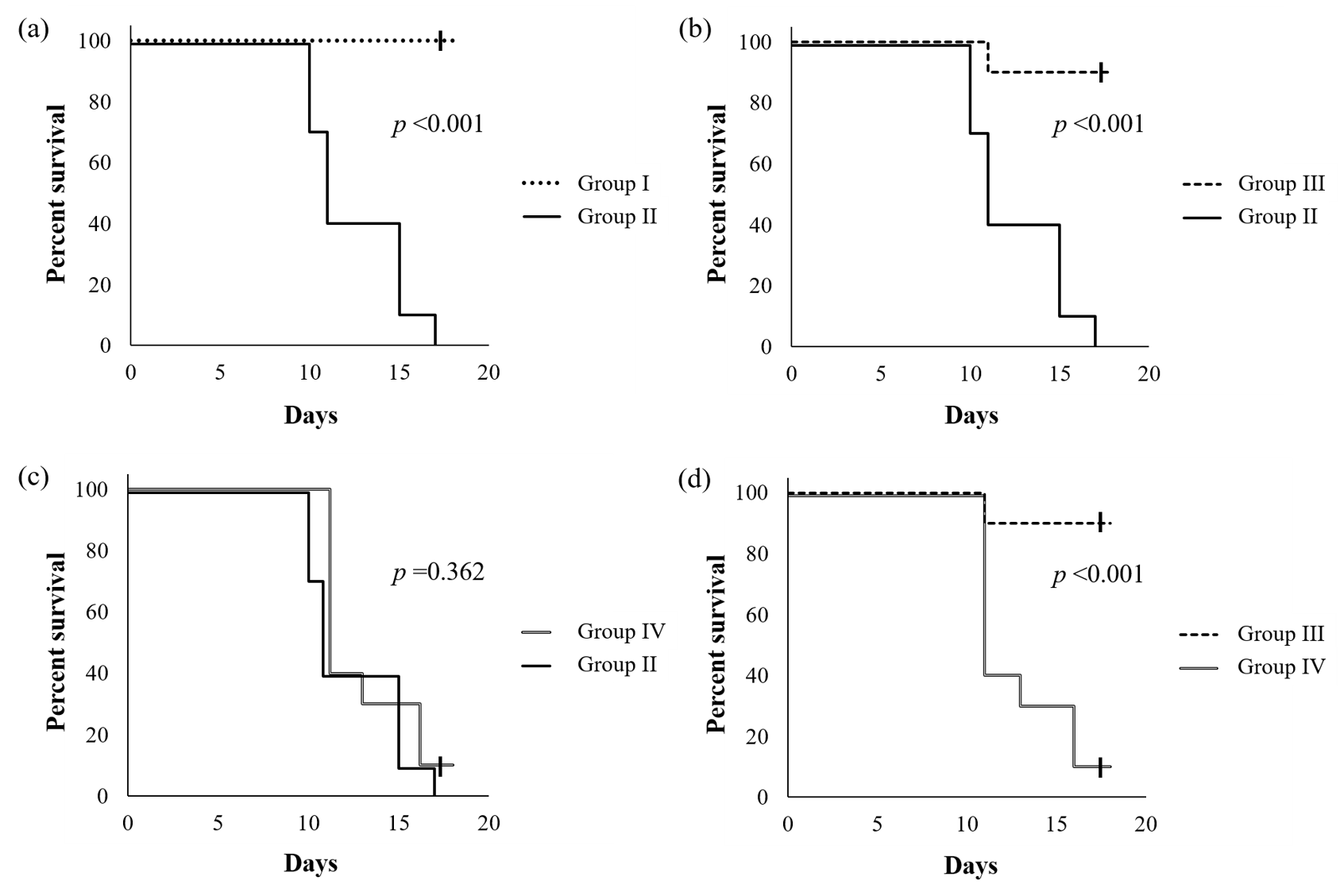
Figure 1 shows the survival curves of mice reared using an ethanol-containing diet supplemented with or without SN13T cells. When compared with a group without the intake of ethanol, the cumulative survival rate of the ethanol-intake group without the added live SN13T cells was significantly decreased (p < 0.001, Figure 1a), and the mice died within 17 days. However, with simultaneous administration of live SN13T cells, the survival rate of the mice did not decrease when compared with a group receiving no alcohol (Figure 1b), strictly, although only one mouse died. In contrast, the survival rate (p = 0.362, Figure 1c) was not improved under supplementation with the heat-killed cells, indicating that there is an obvious difference between the live and heat-killed cell groups (p < 0.001, Figure 1d). Thus, the administration of live SN13T cells is essential for improving alcohol-poisoning symptoms.
Figure 1. The Kaplan–Meier survival curves of C57BL/6J mice fed an ethanol-containing diet with or without the addition of live SN13T cells. The group fed without ethanol was also compared. The p-values were calculated using the log-rank test. Group I, which was fed only an L10015 diet without the administration of ethanol; group II, which was fed an L10016 diet with ethanol; groups III and IV, which were fed with the simultaneous administration of ethanol and the live or heat-killed SN13T cells, respectively, in the L10016 diet. Each graph shows the comparison of survival rate (%) between Groups I and II (a), II and III (b), II and IV (c), and III and IV (d), respectively.
We also analyzed the intestinal microbiota of mice fed an ethanol-containing diet supplemented with or without live SN13T cells. The intestinal microbiota from mice fed with the ethanol-containing diet without bacterial cells was obviously different from that of mice fed with a diet supplemented without both ethanol and the bacterial cells. In the cecum, the ratios of Akkermansia, Allobaculum, and Paraprevotellaceae were remarkably decreased by the administration of ethanol, but these phenomena were disappeared with the simultaneous administration with live SN13T cells. On the other hand, the ratio of an RF32 order was notable increased in ethanol-fed group. Interestingly, when live SN13T cells were administered simultaneously with ethanol, the population of RF32 was clearly decreased.
2. Discussion
The metabolite profiles in the cecum were clearly different between ethanol-fed and nonethanol-fed groups. Change in the cecum components, which has a buffering effect against alteration of the intestinal microbiota, may affect the homeostasis. The metabolite analysis showed that putrefactive amines, such as tyramine and cadaverine, were increased in the ethanol-fed group. In addition to putrefactive amines, a rise in the volume of isovaleric acid and valeric acid content was also observed. These compounds are generated by the hydrolysis of protein in the putrefaction process of tissues and organs and cause an offensive odor. These results reveal that the production of compounds related to putrefaction was promoted during ethanol abuse. Therefore, that putrefaction and the observed mouse death are considered to be due to ethanol abuse. The undesirable effects were repressed by the intake of live SN13T cells. Since it is not evaluated at this moment what species and reactions are involved in the putrefaction with ethanol, further studies on the function of indigenous bacteria such as the RF32 order to intestinal microbiota, is necessary to confirm the mechanisms. As a clue, there is an obvious relationship among colonic damage, inflammation, and abundance of the RF32 order[15]. The research supports our hypothesis that the intake of live SN13T cells restores the inflammation caused by ethanol abuse via changing the intestinal microbiota.
We have demonstrated that alcohol-poisoning symptoms are improved by the oral administration of live SN13T cells but not by the administration of heat-killed cells. Based on these results, it is presumed that live SN13T cells or their collaboration with other intestinal bacteria may generate bioactive compounds. The bioactive compounds may cooperatively inhibit the synthesis of harmful products in the intestinal tract. Some research has shown that a change in the intestinal microbiota is involved in lifestyle and mental diseases[16][17]. It is demonstrated that live SN13T cells are significant for maintaining intestinal microbiota balance and restoring from the symptoms of alcoholism.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21051896
References
- Mary Ellen Sanders; Probiotics: Definition, Sources, Selection, and Uses. Clinical Infectious Diseases 2008, 46, S58-S61, 10.1086/523341.
- Oskar Adolfsson; Simin Nikbin Meydani; Robert M Russell; Yogurt and gut function.. The American Journal of Clinical Nutrition 2004, 80, 245-256, .
- M.L Cross; L.M. Stevenson; H. S. Gill; Anti-allergy properties of fermented foods: an important immunoregulatory mechanism of lactic acid bacteria?. International Immunopharmacology 2001, 1, 891-901, 10.1016/s1567-5769(01)00025-x.
- Martine Heyman; Effect of lactic acid bacteria on diarrheal diseases.. Journal of the American College of Nutrition 2000, 19, 137S-146S, 10.1080/07315724.2000.10718084.
- Simin Nikbin Meydani; Woel-Kyu Ha; Immunologic effects of yogurt.. The American Journal of Clinical Nutrition 2000, 71, 861-872, 10.1093/ajcn/71.4.861.
- S. Parvez; K.A. Malik; S. Ah Kang; Hong-Yeoul Kim; Probiotics and their fermented food products are beneficial for health. Journal of Applied Microbiology 2006, 100, 1171-1185, 10.1111/j.1365-2672.2006.02963.x.
- Yanping Wang; Nv Xu; Aodeng Xi; Zaheer Ahmed; Bin Zhang; Xiaojia Bai; Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Applied Microbiology and Biotechnology 2009, 84, 341-347, 10.1007/s00253-009-2012-x.
- T.D.T. Nguyen; J.H. Kang; M.S. Lee; Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. International Journal of Food Microbiology 2007, 113, 358-361, 10.1016/j.ijfoodmicro.2006.08.015.
- M.T. Liong; N.P. Shah; Effects of a Lactobacillus casei Synbiotic on Serum Lipoprotein, Intestinal Microflora, and Organic Acids in Rats. Journal of Dairy Science 2006, 89, 1390-1399, 10.3168/jds.s0022-0302(06)72207-x.
- Hekui Jin; Fumiko Higashikawa; Masafumi Noda; Xingrong Zhao; Yasuyuki Matoba; Takanori Kumagai; Masanori Sugiyama; Establishment of an in vitro Peyer's patch cell culture system correlative to in vivo study using intestine and screening of lactic acid bacteria enhancing intestinal immunity.. Biological & Pharmaceutical Bulletin 2010, 33, 289-293, 10.1248/bpb.33.289.
- Xingrong Zhao; Fumiko Higashikawa; Masafumi Noda; Yusuke Kawamura; Yasuyuki Matoba; Takanori Kumagai; Masanori Sugiyama; The Obesity and Fatty Liver Are Reduced by Plant-Derived Pediococcus pentosaceus LP28 in High Fat Diet-Induced Obese Mice. PLOS ONE 2012, 7, e30696, 10.1371/journal.pone.0030696.
- F Higashikawa; M Noda; T Awaya; N Danshiitsoodol; Y Matoba; T Kumagai; M Sugiyama; Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: a randomized, double-blind, placebo-controlled clinical trial. European Journal of Clinical Nutrition 2016, 70, 582-587, 10.1038/ejcn.2016.17.
- Masafumi Noda; Nasrin Sultana; Ikue Hayashi; Mitsuhiro Fukamachi; Masanori Sugiyama; Exopolysaccharide Produced by Lactobacillus paracasei IJH-SONE68 Prevents and Improves the Picryl Chloride-Induced Contact Dermatitis.. Molecules 2019, 24, 2970, 10.3390/molecules24162970.
- Fumiko Higashikawa; Masafumi Noda; Tomokazu Awaya; Kazuhiro Nomura; Hirotaka Oku; Masanori Sugiyama; Improvement of constipation and liver function by plant-derived lactic acid bacteria: A double-blind, randomized trial. Nutrition 2010, 26, 367-374, 10.1016/j.nut.2009.05.008.
- Josué L. Castro-Mejía; Maja Jakesevic; Łukasz Krych; Dennis S. Nielsen; Lars H. Hansen; Bodil C. Sondergaard; Peter H. Kvist; Axel K. Hansen; Thomas L. Holm; Treatment with a Monoclonal Anti-IL-12p40 Antibody Induces Substantial Gut Microbiota Changes in an Experimental Colitis Model. Gastroenterology Research and Practice 2016, 2016, 1-12, 10.1155/2016/4953120.
- Chikako Shimokawa; Seiji Obi; Mioko Shibata; Alex Olia; Takashi Imai; Kazutomo Suzue; Hajime Hisaeda; Suppression of Obesity by an Intestinal Helminth through Interactions with Intestinal Microbiota.. Infection and Immunity 2019, 87, e00042-19, 10.1128/IAI.00042-19.
- Shan Liang; Xiaoli Wu; Jin Feng; Gut-Brain Psychology: Rethinking Psychology From the Microbiota–Gut–Brain Axis. Frontiers in Integrative Neuroscience 2018, 12, 33, 10.3389/fnint.2018.00033.