Since the emergence of the COVID-19 pandemic at the end of 2019, a massive vaccination campaign has been undertaken rapidly and worldwide. Like other vaccines, the COVID-19 vaccine is not devoid of side effects. Typically, the adverse side effects of vaccination include transient headache, fever, and myalgia. Endocrine organs are also affected by adverse effects. The major SARS-CoV-2 vaccine-associated endocrinopathies reported since the beginning of the vaccination campaign are thyroid and pancreas disorders. SARS-CoV-2 vaccine-induced pituitary diseases have become more frequently described in the literature.
- pituitary
- SARS-CoV-2
- COVID-19
- vaccine
- hypophysitis
- apoplexy
- ASIA syndrome
- vaccine-induced thrombotic thrombocytopenia
- VITT
1. Introduction
2. Hypophysitis
2.1. Pathogenesis
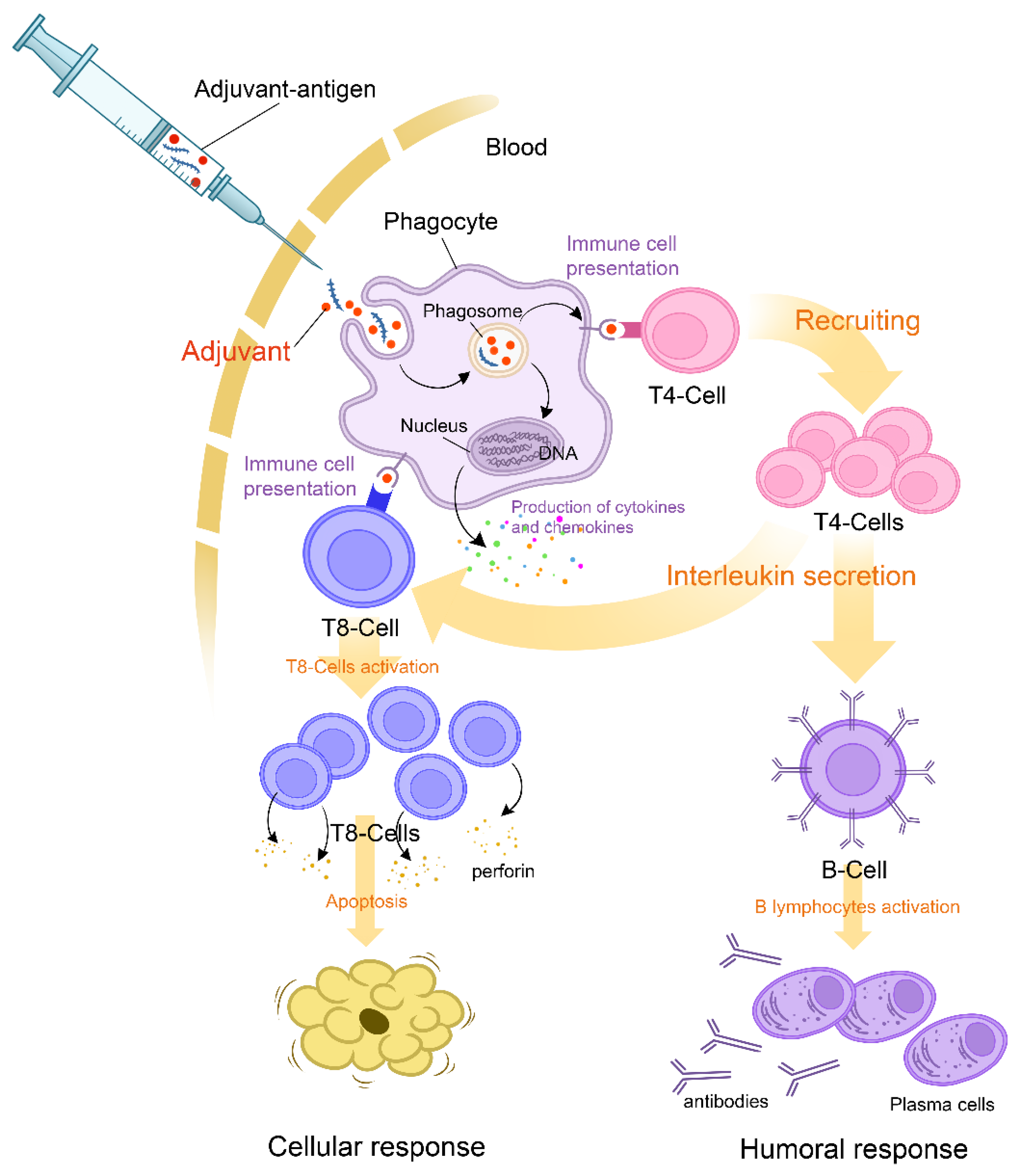
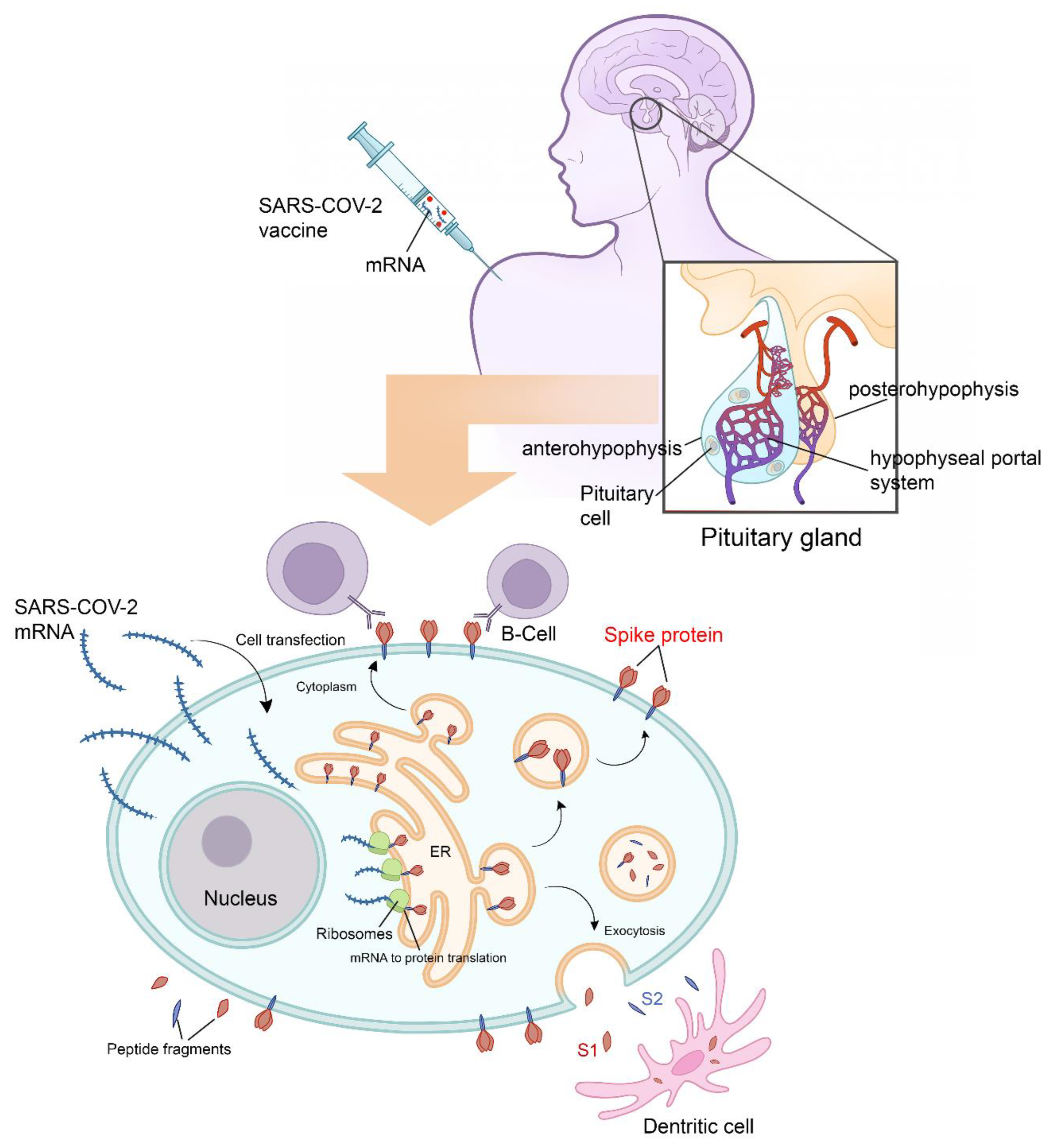
2.2. Clinical Presentations
2.3. Treatment
3. Pituitary Apoplexy
3.1. Pathogenesis
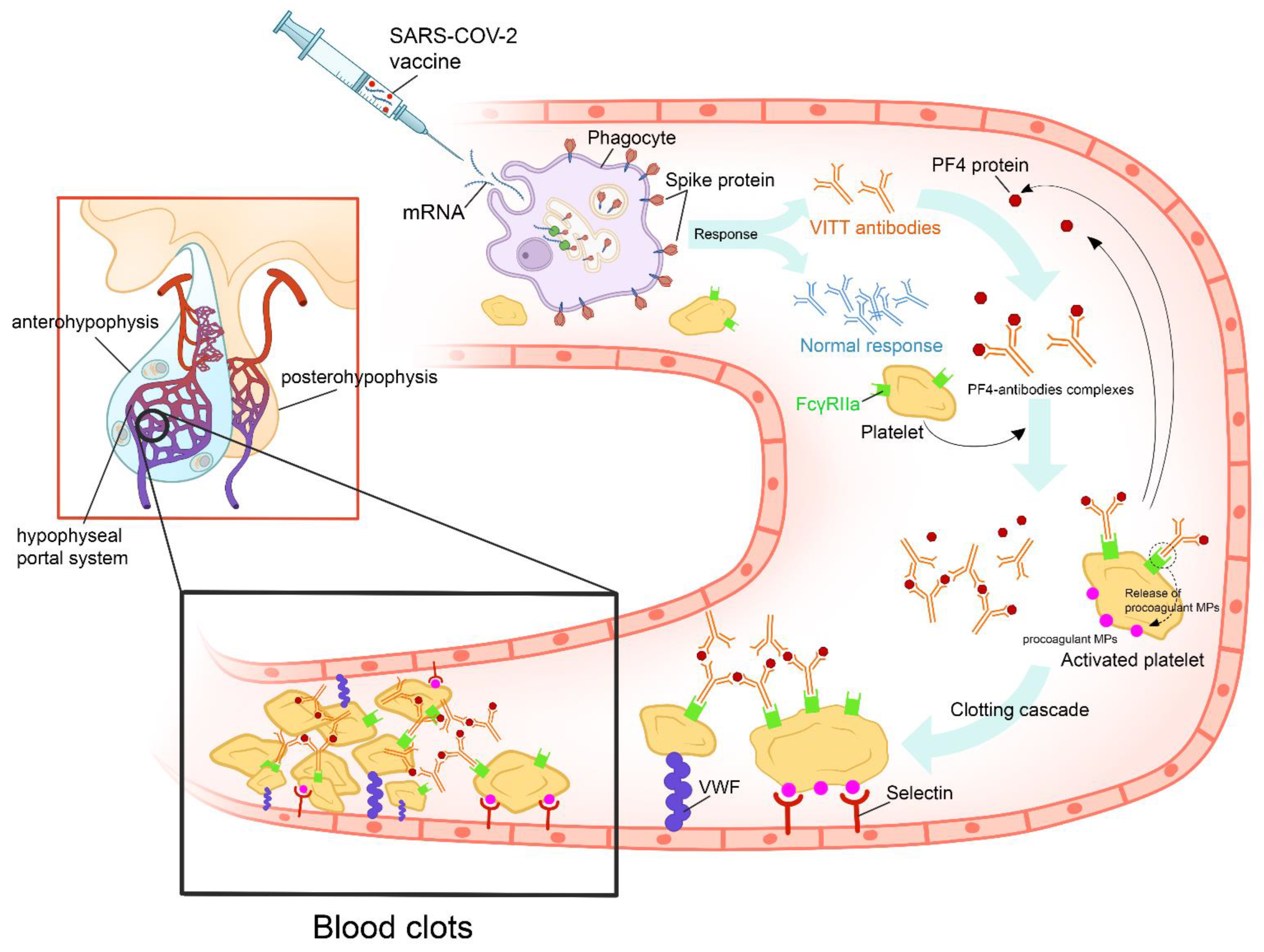
3.2. Clinical Presentations
3.3. Treatment
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10122004
References
- Yu, J.; Chai, P.; Ge, S.; Fan, X. Recent Understandings Toward Coronavirus Disease 2019 (COVID-19): From Bench to Bedside. Front. Cell Dev. Biol. 2020, 8, 476.
- Wong, R.S.Y. The SARS-CoV-2 Outbreak: An Epidemiological and Clinical Perspective. SN Compr. Clin. Med. 2020, 2, 1983–1991.
- Johnson, K.D.; Harris, C.; Cain, J.K.; Hummer, C.; Goyal, H.; Perisetti, A. Pulmonary and Extra-Pulmonary Clinical Manifestations of COVID-19. Front. Med. 2020, 7, 526.
- Rabail, R.; Ahmed, W.; Ilyas, M.; Rajoka, M.S.R.; Hassoun, A.; Khalid, A.R.; Khan, M.R.; Aadil, R.M. The Side Effects and Adverse Clinical Cases Reported after COVID-19 Immunization. Vaccines 2022, 10, 488.
- Zhao, Y.; Wu, X. Influence of COVID-19 vaccines on endocrine system. Endocrine 2022, 78, 241–246.
- Ku, C.R.; Jung, K.Y.; Ahn, C.H.; Moon, J.S.; Lee, J.H.; Kim, E.H.; Kwon, H.; Kim, H.K.; Suh, S.; Hong, S.; et al. COVID-19 Vaccination for Endocrine Patients: A Position Statement from the Korean Endocrine Society. Endocrinol. Metab. 2021, 36, 757–765.
- Soldevila, B.; Puig-Domingo, M.; Marazuela, M. Basic mechanisms of SARS-CoV-2 infection. What endocrine systems could be implicated? Rev. Endocr. Metab. Disord. 2022, 23, 137–150.
- Young, M.J.; Clyne, C.D.; Chapman, K.E. Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2. J. Endocrinol. 2020, 247, R45–R62.
- Garg, M.K.; Gopalakrishnan, M.; Yadav, P.; Misra, S. Endocrine Involvement in COVID-19: Mechanisms, Clinical Features, and Implications for Care. Indian J. Endocrinol. Metab. 2020, 24, 381–386.
- Lisco, G.; De Tullio, A.; Stragapede, A.; Solimando, A.; Albanese, F.; Capobianco, M.; Giagulli, V.; Guastamacchia, E.; De Pergola, G.; Vacca, A.; et al. COVID-19 and the Endocrine System: A Comprehensive Review on the Theme. J. Clin. Med. 2021, 10, 2920.
- Mirza, S.A.; Sheikh, A.A.E.; Barbera, M.; Ijaz, Z.; Javaid, M.A.; Shekhar, R.; Pal, S.; Sheikh, A.B. COVID-19 and the Endocrine System: A Review of the Current Information and Misinformation. Infect. Dis. Rep. 2022, 14, 184–197.
- Zhang, C.; Zhou, C.; Shi, L.; Liu, G. Perspectives on development of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Hum. Vaccines Immunother. 2020, 16, 2366–2369.
- Zhou, H.; Møhlenberg, M.; Thakor, J.C.; Tuli, H.S.; Wang, P.; Assaraf, Y.G.; Dhama, K.; Jiang, S. Sensitivity to Vaccines, Therapeutic Antibodies, and Viral Entry Inhibitors and Advances To Counter the SARS-CoV-2 Omicron Variant. Clin. Microbiol. Rev. 2022, 35, e0001422.
- Gubbi, S.; Hannah-Shmouni, F.; Verbalis, J.G.; Koch, C.A. Hypophysitis: An update on the novel forms, diagnosis and management of disorders of pituitary inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101371.
- Faje, A. Hypophysitis: Evaluation and Management. Clin. Diabetes Endocrinol. 2016, 2, 15.
- Ach, T.; Wojewoda, P.; Toullet, F.; Ducloux, R.; Avérous, V. Multiple endocrinological failures as a clinical presentation of a metastatic lung adenocarcinoma. Endocrinol. Diabetes Metab. Case Rep. 2020, 2020.
- Day, E.L.; Smith, E.R.; Fehnel, K.P. Single-institution case series of pituitary biopsy for suspected germinoma in the pediatric population: Diagnostic utility, operative risks, and biopsy approaches. Sci. Rep. 2020, 10, 15257.
- Kang, H.; Kim, K.M.; Kim, M.S.; Kim, J.H.; Park, C.K.; Kim, Y.H. Safety of endoscopic endonasal biopsy for the pituitary stalk-hypothalamic lesions. Pituitary 2022, 25, 143–151.
- Jara, L.J.; Vera-Lastra, O.; Mahroum, N.; Pineda, C.; Shoenfeld, Y. Autoimmune post-COVID vaccine syndromes: Does the spectrum of autoimmune/inflammatory syndrome expand? Clin. Rheumatol. 2022, 41, 1603–1609.
- Liang, Z.; Zhu, H.; Wang, X.; Jing, B.; Li, Z.; Xia, X.; Sun, H.; Yang, Y.; Zhang, W.; Shi, L.; et al. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020, 11, 589833.
- Chen, P.-Y.; Wu, B.-J.; Su, M.-C.; Lin, Y.-H.; Chiang, S.-C.; Wu, J.-C.; Chen, T.-J.; Chen, Y.-C. Risk Factors and Incidence Rates of Self-Reported Short-Term Adverse Events of COVID-19 Vaccine Booster Dose. Vaccines 2022, 10, 1115.
- Wilson, B.; Geetha, K.M. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022, 74, 103553.
- Bellamkonda, N.; Lambe, U.P.; Sawant, S.; Nandi, S.S.; Chakraborty, C.; Shukla, D. Immune Response to SARS-CoV-2 Vaccines. Biomedicines 2022, 10, 1464.
- Guo, S.; Wang, C.; Zhang, J.; Tian, Y.; Wu, Q. Diagnosis and management of tumor-like hypophysitis: A retrospective case series. Oncol. Lett. 2016, 11, 1315–1320.
- Caranci, F.; Leone, G.; Ponsiglione, A.; Muto, M.; Tortora, F.; Muto, M.; Cirillo, S.; Brunese, L.; Cerase, A. Imaging findings in hypophysitis: A review. La Radiol. Med. 2020, 125, 319–328.
- Gutenberg, A.; Larsen, J.; Lupi, I.; Rohde, V.; Caturegli, P. A radiologic score to distinguish autoimmune hypophysitis from nonsecreting pituitary adenoma preoperatively. AJNR Am. J. Neuroradiol. 2009, 30, 1766–1772.
- Mutter, C.M.; Smith, T.; Menze, O.; Zakharia, M.; Nguyen, H. Diabetes Insipidus: Pathogenesis, Diagnosis, and Clinical Management. Cureus 2021, 13, e13523.
- Taieb, A.; Maha, K.N.; El Abed, Y.H.; Beizig, A.M.; Chadli, M.C.; Ach, K. Macroprolactinemia and Empty Sella Syndrome. Pan Afr. Med. J. 2017, 27, 278.
- Ach, T.; Kammoun, F.; El Fekih, H.; Slama, N.B.H.; Kahloun, S.; Fredj, F.B. Central diabetes insipidus revealing a hypophysitis induced by SARS-CoV-2 vaccine. Therapie 2022.
- Honegger, J.; Buchfelder, M.; Schlaffer, S.; Droste, M.; Werner, S.; Strasburger, C.; Störmann, S.; Schopohl, J.; Kacheva, S.; Deutschbein, T.; et al. Treatment of Primary Hypophysitis in Germany. J. Clin. Endocrinol. Metab. 2015, 100, 3460–3469.
- Boellis, A.; di Napoli, A.; Romano, A.; Bozzao, A. Pituitary apoplexy: An update on clinical and imaging features. Insights Into Imaging 2014, 5, 753–762.
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362.
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101.
- Lai, K.Y.; Au, S.Y.; Fong, K.M. Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 385, e11.
- Bissola, A.-L.; Daka, M.; Arnold, D.M.; Smith, J.W.; Moore, J.C.; Clare, R.; Ivetic, N.; Kelton, J.G.; Nazy, I. The clinical and laboratory diagnosis of vaccine-induced immune thrombotic thrombocytopenia. Blood Adv. 2022, 6, 4228–4235.
- Mungmunpuntipantip, R.; Wiwanitkit, V. Pituitary apoplexy and COVID-19 vaccination. Med. Clin. (Engl. Ed.) 2022, 159, e11.
- Ziogas, A.; Netea, M.G. Trained immunity-related vaccines: Innate immune memory and heterologous protection against infections. Trends Mol. Med. 2022, 28, 497–512.
- Taieb, A.; Asma, B.A.; Ghada, S.; Yosra, H.; Maha, K.; Molka, C.; Amel, M.; Koussay, A. Increased intracranial pressure due to chronic weight lifting exercises as a hypothesis of partial empty sella syndrome in an elite athlete. Med. Hypotheses 2022, 167, 110951.
- Glezer, A.; Bronstein, M.D. Pituitary apoplexy: Pathophysiology, diagnosis and management. Arch. Endocrinol. Metab. 2015, 59, 259–264.
- Briet, C.; Salenave, S.; Bonneville, J.F.; Laws, E.R.; Chanson, P. Pituitary Apoplexy. Endocr. Rev. 2015, 36, 622–645.
- Biagetti, B.; Simò, R. Pituitary Apoplexy: Risk Factors and Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 8721.
- Leyer, C.; Castinetti, F.; Morange, I.; Gueydan, M.; Oliver, C.; Conte-Devolx, B.; Dufour, H.; Brue, T. A conservative management is preferable in milder forms of pituitary tumor apoplexy. J. Endocrinol. Investig. 2011, 34, 502–509.
- Goyal, P.; Utz, M.; Gupta, N.; Kumar, Y.; Mangla, M.; Gupta, S.; Mangla, R. Clinical and imaging features of pituitary apoplexy and role of imaging in differentiation of clinical mimics. Quant. Imaging Med. Surg. 2018, 8, 219–231.
- Albani, A.; Ferraù, F.; Angileri, F.; Esposito, F.; Granata, F.; Ferreri, F.; Cannavò, S. Multidisciplinary Management of Pituitary Apoplexy. Int. J. Endocrinol. 2016, 2016, 7951536.
- Kyle, C.A.; Laster, R.A.; Burton, E.M.; Sanford, R.A. Subacute pituitary apoplexy: MR and CT appearance. J. Comput. Assist. Tomogr. 1990, 14, 40–44.
- Khaldi, S.; Saad, G.; Elfekih, H.; Ben Abdelkrim, A.; Ach, T.; Kacem, M.; Chaieb, M.; Marroufi, A.; Hasni, Y.; Ach, K.; et al. Pituitary apoplexy of a giant prolactinoma during pregnancy. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2021, 37, 863–866.
- Almeida, J.P.; Sanchez, M.M.; Karekezi, C.; Warsi, N.; Fernández-Gajardo, R.; Panwar, J.; Mansouri, A.; Suppiah, S.; Nassiri, F.; Nejad, R.; et al. Pituitary Apoplexy: Results of Surgical and Conservative Management Clinical Series and Review of the Literature. World Neurosurg. 2019, 130, e988–e999.
- Gabarin, N.; Arnold, D.M.; Nazy, I.; Warkentin, T.E. Treatment of vaccine-induced immune thrombotic thrombocytopenia (VITT). Semin. Hematol. 2022, 59, 89–96.
- Singh, B.; Kanack, A.; Bayas, A.; George, G.; Abou-Ismail, M.Y.; Kohlhagen, M.; Christ, M.; Naumann, M.; Moser, K.; Smock, K.; et al. Anti-PF4 VITT antibodies are oligoclonal and variably inhibited by heparin. Medrxiv Prepr. Serv. Health Sci. 2021.
