Mycotoxins are natural metabolites produced by fungi that contaminate food and feed worldwide. They can pose a threat to human and animal health, mainly causing chronic effects, e.g., immunotoxic and carcinogenic. Due to climate change, an increase in European population exposure to mycotoxins is expected to occur, raising public health concerns. This urges researchers to assess the current human exposure to mycotoxins in Europe to allow monitoring exposure and prevent future health impacts. The mycotoxins deoxynivalenol (DON) and fumonisin B1 (FB1) were considered as priority substances to be studied within the European Human Biomonitoring Initiative (HBM4EU) to generate knowledge on internal exposure and their potential health impacts.
- human biomonitoring
- risk assessment
- HBM4EU
- human health
- mycotoxins exposure
1. Human Exposure to Mycotoxins
2. Mycotoxins in the Context of the HBM4EU Initiative
|
Policy Questions on Mycotoxins under HBM4EU |
|
|---|---|
|
Hazard assessment |
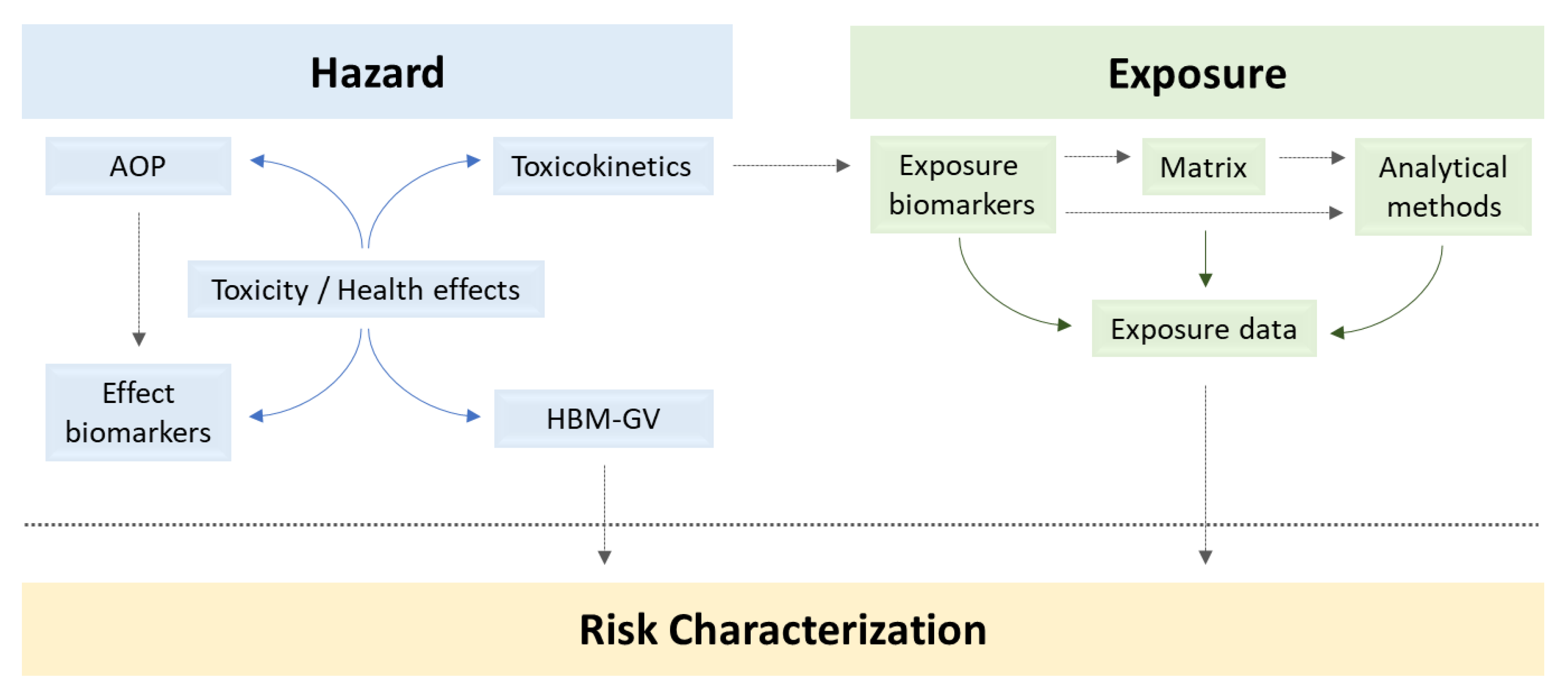
Are there toxicokinetics data for the target mycotoxins and what are their limitations? What are the key events that determine the chronic health effects of the target mycotoxins? What are the most frequent AOP-based effect biomarkers for the prioritized mycotoxins? Is it possible to set HBM guidance values for the target mycotoxins? |
|
Exposure assessment |
Are there validated and harmonised analytical methods to assess the target mycotoxins’ exposure? Which are the current exposure levels of the European population to DON and FB1? Does the exposure to the target mycotoxins differ among different population groups? Which are the main exposure determinants? |
|
Risk characterization |
Is the risk associated with human exposure to these mycotoxins characterized? |
3. Prioritized Mycotoxins: Occurrence, Toxicological Properties and Exposure Thresholds
This entry is adapted from the peer-reviewed paper 10.3390/toxins14120826
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516.
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public Health Impacts of Foodborne Mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372.
- Köppen, R.; Koch, M.; Siegel, D.; Merkel, S.; Maul, R.; Nehls, I. Determination of Mycotoxins in Foods: Current State of Analytical Methods and Limitations. Appl. Microbiol. Biotechnol. 2010, 86, 1595–1612.
- Louro, H.; Heinälä, M.; Bessems, J.; Buekers, J.; Vermeire, T.; Woutersen, M.; Van Engelen, J.; Borges, T.; Rousselle, C.; Ougier, E.; et al. International Journal of Hygiene and Human Biomonitoring in Health Risk Assessment in Europe: Current Practices and Recommendations for the Future. Int. J. Hyg. Environ. Health 2019, 222, 727–737.
- Viegas, S.; Viegas, C.; Oppliger, A. Occupational Exposure to Mycotoxins: Current Knowledge and Prospects. Ann. Work Expo. Health 2018, 62, 923–941.
- European Commission (EU). Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. EFSA J. 2006, 364, 5–24.
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on the Risks for Human and Animal Health Related to the Presence of Modified Forms of Certain Mycotoxins in Food and Feed. EFSA J. 2014, 12, 3916.
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070.
- Ekwomadu, T.I.; Akinola, S.A.; Mwanza, M. Fusarium Mycotoxins, Their Metabolites (Free, Emerging, and Masked), Food Safety Concerns, and Health Impacts. Int. J. Environ. Res. Public Health 2021, 18, 11741.
- Alvito, P.; Barcelo, J.; De Meester, J.; Rito, E.; Suman, M. Mitigation of Mycotoxins during Food Processing: Sharing Experience among Europe and South East Asia. Sci. Technol. Cereal. Oils Foods 2021, 29, 59–70.
- Miraglia, M.; Marvin, H.J.P.; Kleter, G.A.; Battilani, P.; Brera, C.; Coni, E.; Cubadda, F.; Croci, L.; De Santis, B.; Dekkers, S.; et al. Climate Change and Food Safety: An Emerging Issue with Special Focus on Europe. Food Chem. Toxicol. 2009, 47, 1009–1021.
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B 1 Contamination in Maize in Europe Increases Due to Climate Change. Sci. Rep. 2016, 6, 1–7.
- Assunção, R.; Martins, C.; Viegas, S.; Viegas, C.; Jakobsen, L.S.; Pires, S.; Alvito, P. Climate Change and the Health Impact of Aflatoxins Exposure in Portugal–an Overview. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 1610–1621.
- Alvito, P.; Assunção, R. Climate Change and the Impact on Aflatoxin Contamination in Foods: Where Are We and What Should Be Expected? In Aflatoxins in Food; Springer: Cham, Switzerland, 2021; ISBN 9783030857615.
- Zingales, V.; Taroncher, M.; Martino, P.A.; Caloni, F. Climate Change and Effects on Molds and Mycotoxins. Toxins 2022, 14, 445.
- Bizjak, T.; Capodiferro, M.; Deepika, D.; Dinçkol, Ö.; Dzhedzheia, V.; Lopez-Suarez, L.; Petridis, I.; Runkel, A.A.; Schultz, D.R.; Kontić, B. Human Biomonitoring Data in Health Risk Assessments Published in Peer-Reviewed Journals between 2016 and 2021: Confronting Reality after a Preliminary Review. Int. J. Environ. Res. Public Health 2022, 19, 3362.
- Choi, J.; Aarøe Mørck, T.; Polcher, A.; Knudsen, L.E.; Joas, A. Review of the State of the Art of Human Biomonitoring for Chemical Substances and Its Application to Human Exposure Assessment for Food Safety. EFSA Support. Publ. 2015, 12, 724.
- World Health Organisation (WHO). Biomarkers and Risk Assessment: Concepts and Principles. Environmental Health Criteria 155; WHO Library Cataloguing in Publication Data: Vammala, Finland, 1993; ISBN 9241571551.
- European Commission. EUR-Lex–52004DC0416–EN 2004. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52004DC0416&from=EN (accessed on 4 July 2022).
- Ganzleben, C.; Antignac, J.P.; Barouki, R.; Castaño, A.; Fiddicke, U.; Klánová, J.; Lebret, E.; Olea, N.; Sarigiannis, D.; Schoeters, G.R.; et al. Human Biomonitoring as a Tool to Support Chemicals Regulation in the European Union. Int. J. Hyg. Environ. Health 2017, 220, 94–97.
- Ormsby, J.-N.; Lecoq, P.; Ougier, E.; Rousselle, C.; Ganzleben, C. HBM4EU—Prioritisation, Strategy and Criteria, Deliverable Report D4.3; 2017. Available online: https://www.hbm4eu.eu/work-packages/deliverable-4-3-prioritisation-strategy-and-criteria/ (accessed on 12 July 2022).
- Ougier, E.; Ganzleben, C.; Lecoq, P.; Bessems, J.; David, M.; Schoeters, G.; Lange, R.; Meslin, M.; Uhl, M.; Kolossa-gehring, M.; et al. Chemical Prioritisation Strategy in the European Human Biomonitoring Initiative (HBM4EU)—Development and Results. Int. J. Hyg. Environ. Health 2021, 236, 113778.
- Schoeters, G.; Rosa, L.; Kolossa, M.; Barouki, R.; Tarroja, E.; Uhl, M.; Klanova, J.; Melymuk, L.; Horvat, M.; Bocca, B.; et al. HBM4EU—Scoping Documents for 2021 for the First and Second Second Round HBM4EU Priority Substances Deliverable Report D4.9. 2021. Available online: https://www.hbm4eu.eu/work-packages/deliverable-4-9-scoping-documents-for-2021-for-the-first-and-second-second-round-hbm4eu-priority-substances/ (accessed on 12 July 2022).
- Schoeters, G.; Lange, R.; Laguzzi, F.; Kadikis, N.; Wasowicz, W.; Santonen, T.; Mahiout, S.; Rudnai, P.; Katsonouri-Sazeides, A.; Alvito, P.; et al. HBM4EU—Scoping Documents for the Second Round Priority Substances Deliverable Report D4.6; 2019. Available online: https://www.hbm4eu.eu/work-packages/deliverable-4-6-scoping-documents-for-the-second-round-priority-substances/ (accessed on 12 July 2022).
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse Outcome Pathways: A Conceptual Framework to Support Ecotoxicology Research and Risk Assessment. Environ. Toxicol. Chem. 2010, 29, 730–741.
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; et al. Risks to Human and Animal Health Related to the Presence of Deoxynivalenol and Its Acetylated and Modified Forms in Food and Feed. EFSA J. 2017, 15, 4718.
- Ndossi, D.G.; Frizzell, C.; Tremoen, N.H.; Fæste, C.K.; Verhaegen, S.; Dahl, E.; Eriksen, G.S.; Sørlie, M.; Connolly, L.; Ropstad, E. An in Vitro Investigation of Endocrine Disrupting Effects of Trichothecenes Deoxynivalenol (DON), T-2 and HT-2 Toxins. Toxicol. Lett. 2012, 214, 268–278.
- IARC. Some Naturally Occurring Susbtances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; ISBN 9283212568.
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Appropriateness to Set a Group Health-Based Guidance Value for Fumonisins and Their Modified Forms. EFSA J. 2018, 16, 5172.
- IARC. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC: Lyon, France, 2002; Volume 82, ISBN 9789283215875.
