Zinc–iron redox flow batteries (ZIRFBs) possess intrinsic safety and stability and have low electrolyte cost. ZBRFB refers to an RFB in which zinc is used as the electrochemically active substance in the electrolyte solutions. The zinc electrode has a reversible anode potential. Zinc ions are stable in both alkaline and acidic environments, even in a neutral electrolyte, and the electrochemical reaction rate is relatively fast.
- zinc–iron
- redox flow battery
- zinc dendrite
- energy storage
- large scale
- carbon electrode
- ion exchange membrane
- electrolyte design
- areal capacity
1. Introduction
2. Characteristics of ZIRFB
2.1. The Basic Principle of ZIRFB
2.2. Wide pH Range
Metallic Zn can be electroplated in four various ways with different forms of reactants in the solution depending on the pH from 0 to 16. The relevant reaction equations are as follows:
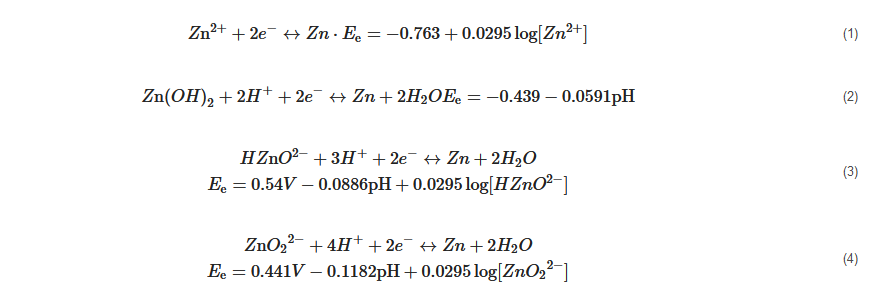
Unlike other RFBs, the electrolyte of ZIRFB can work in a wide pH range. A higher pH value is conducive to the dissolution and deposition of metallic Zn, despite that the Fe2+/Fe3+ redox couple tends to precipitate more easily at high pH. Hence, the appropriate pH range is very important. According to the difference in electrolyte acidity and alkalinity, ZIRFBs are normally divided into three types: alkaline, acidic, and neutral ZIRFBs.
In alkaline ZIRFB, zinc and ferricyanide are used as active substances in the anolyte and catholyte, respectively [51]. The system possesses the electrolyte with relatively low cost and high open-circuit voltage (OCV) of 1.74 V. In the discharge state, the anode side is transformed from Zn to zincate solution (alkaline), while the cathode side ferrocyanide is formed from the previous ferricyanide. When charging, it is the opposite process, which is a reversible reaction compared to the discharge process. However, the cycle performance of the ZIRFB is poor due to the issue of zinc dendrites in the alkaline medium.
In theory, the acidic ZIRFB (Ecell = 1.53 V) can have a higher energy density [54]. However, in the acidic ZIRFB, the excessive acidity of the solution will affect the deposition of zinc and the hydrolysis of the Fe2+/Fe3+ pair, thus, the hydrogen evolution reaction (HER) is prone to occur. For an acidic system with HAc/NaAc as the buffer solution to keep the pH value of the negative electrolyte between 2–6, a high CE (coulombic/current efficiency) can be realized [53].
Compared with alkaline and acidic systems, the neutral ZIRFB system (Ecell = 1.43 V) is mild and non-corrosive, which has lower requirements for the membrane/separator and other components [44]. The neutral ZIRFB has a lower battery cost than the other two systems, to a certain extent. Nevertheless, regarding the neutral ZIRFB system, it also has to be taken into account that the hydrolysis of Fen+ ions may lead to the decline of battery cycle performance, which is one of the primary challenges for this type of battery.
2.3. Zinc Dendrites
2.4. Fe(III) Hydrolysis
3. Research Status of Several Key Problems in ZIRFBs
According to the characteristics of ZIRFBs, the key problems need to be improved including Fe(III) hydrolysis suppression and zinc dendrite prevention, which address the electrode, membrane, and electrolyte optimization, correspondingly.
3.1. Zinc Dendrite Prevention
3.1.1. The 3D Electrode
3.1.2. Improving Membrane/Separator
3.1.3. Adding Additives to the Electrolyte
3.1.4. Flow Field Regulation
3.2. Fe(III) Hydrolysis Suppression
3.3. Electrolyte Optimization
3.3.1. Concentration and Additives
By optimizing the composition of the electrolyte, Yuan et al. made the concentration of the Fe(CN)63−/Fe(CN)64− redox couples achieve 1 mol L−1, far exceeding the previously reported concentration (0.4 mol L−1) [51]. The high concentration of active redox couples enables the system with a high-energy density. The battery can realize 500 cycles of charge–discharge cycling under 80 and 160 mA cm−2, and still maintain an EE over 80% and CE over 99% at 160 mA cm−2. The results verified the outstanding stability of this system. Most important of all, the functionality of this work is further verified by assembling a kW battery stack at a capital cost of less than USD 90 per kWh.
3.3.2. Zinc–Bromide Complexation
3.3.3. pH
3.3.4. Mix System
Abbreviations
| RFBs | redox flow batteries |
| ZIRFBs | zinc–iron redox flow batteries |
| ZBRFB | zinc-based RFB |
| VRFB | vanadium RFB |
| R.T. | room temperature |
| OCV | open-circuit voltage |
| OCP | open-circuit potential |
| CEM | cationic exchange membrane |
| IEM | ion exchange membrane |
| n-IEMs | non-ionic membranes |
| PES | poly (ether sulfone) |
| PEG | polyethene glycol |
| SPEEK-K | sulfonated polyether ether ketone |
| PBI | polybenzimidazole |
| BMImCl | 1-butyl-3-methylimidazolium chloride |
| CF | carbon felts |
| Z-P/S | zinc plating/stripping |
| CV | cyclic voltammogram |
| EE | energy efficiency |
| CE | current efficiency |
| VE | voltage efficiency |
| HER | hydrogen evolution reaction |
| SOC | state of charge |
| MC | microporous carbon |
| THEED | N, N, N′ N′-Tetra(2- hydroxyethyl) ethylenediamine |
This entry is adapted from the peer-reviewed paper 10.3390/batteries8110202
