Hydroarylation reactions enable the formation of new Csp2–Csp3 or Csp2–Csp2 bonds using aromatic substrates. Its outcome is described as the addition of hydrogen and an aryl group to an unsaturated moiety, resulting in the functionalization of the aromatic Csp2–H bond. Hydroarylation reactions can occur by a direct functionalization via insertion of an unsaturated compound or with the use of pre-activated aryl substrates, usually aryl-iodides or aryl-boronated. Below are reported some example from the scientific production of the last 10 years about the asymmetric hydroarylation of activated aryl portions. In this context, nickel and palladium turned out to be the more-employed metals.
- nickel
- asymmetric hydroarylation
- palladium
1. Palladium
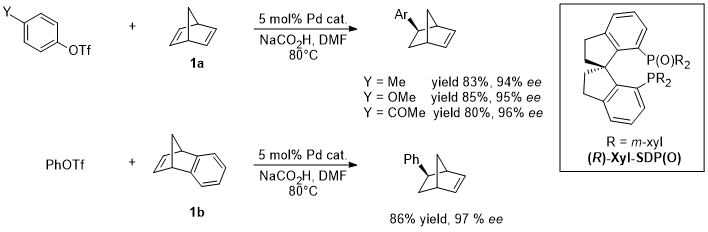
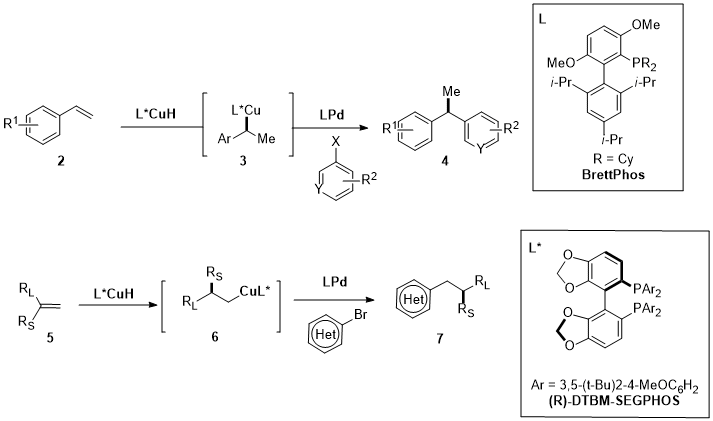
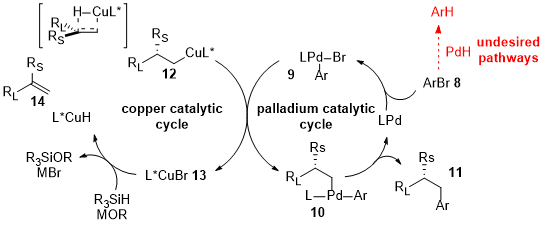
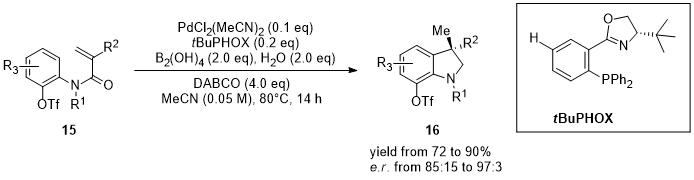
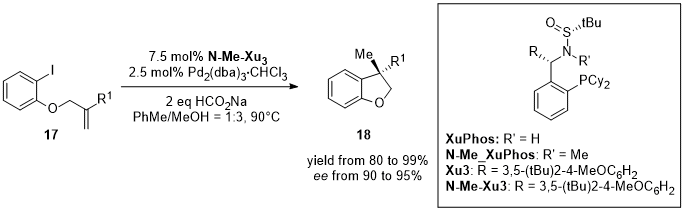
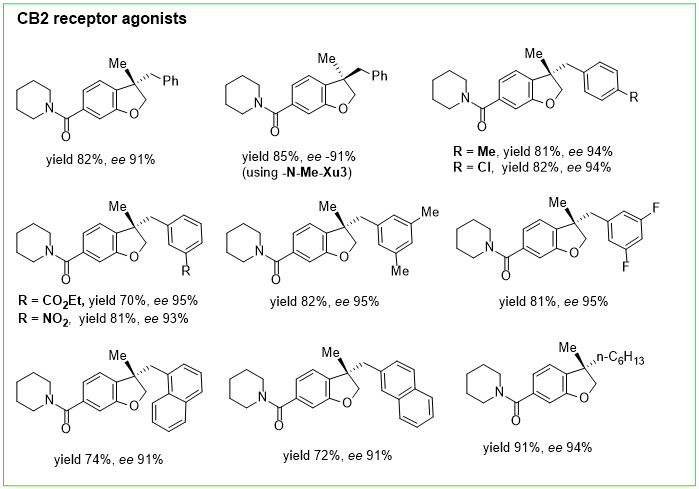
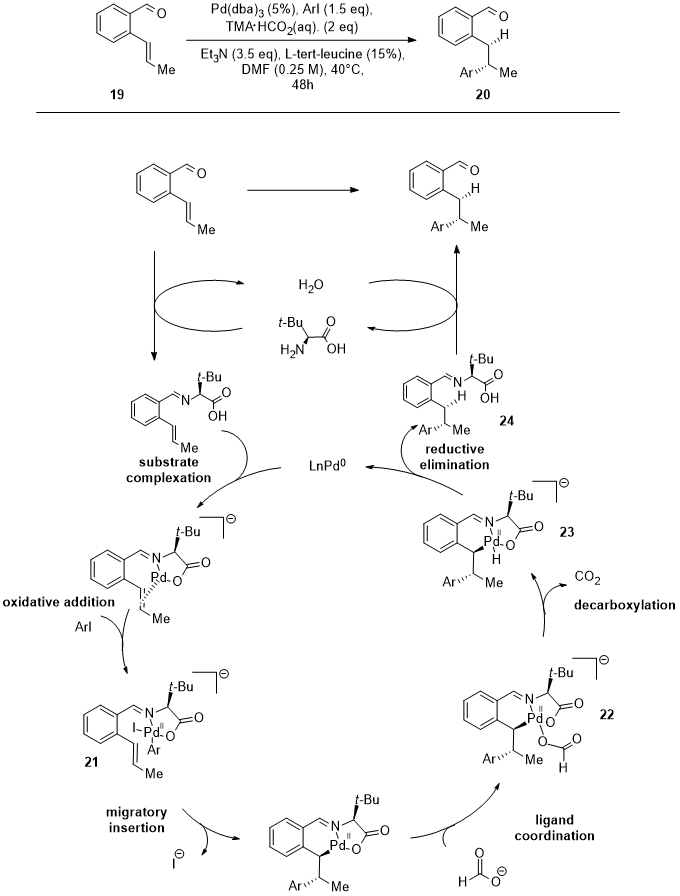
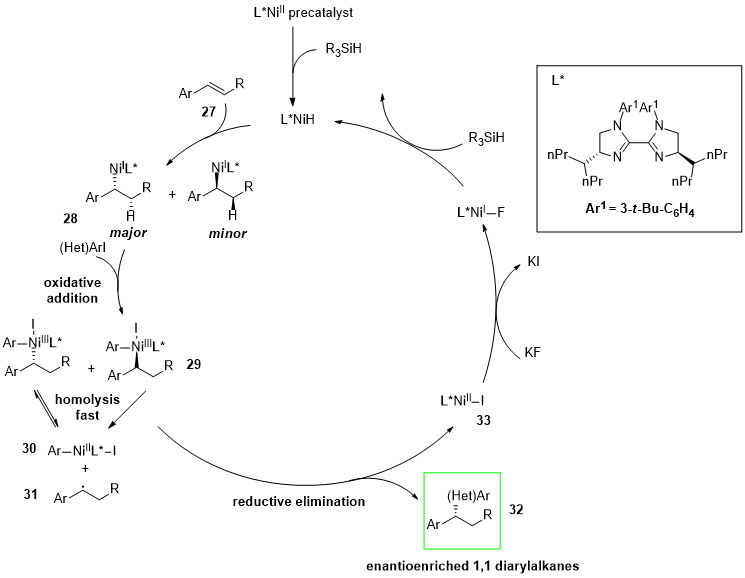
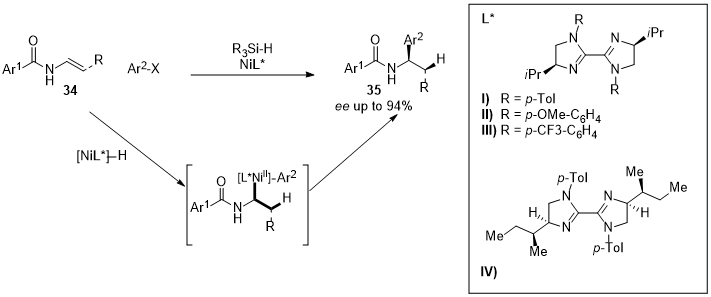
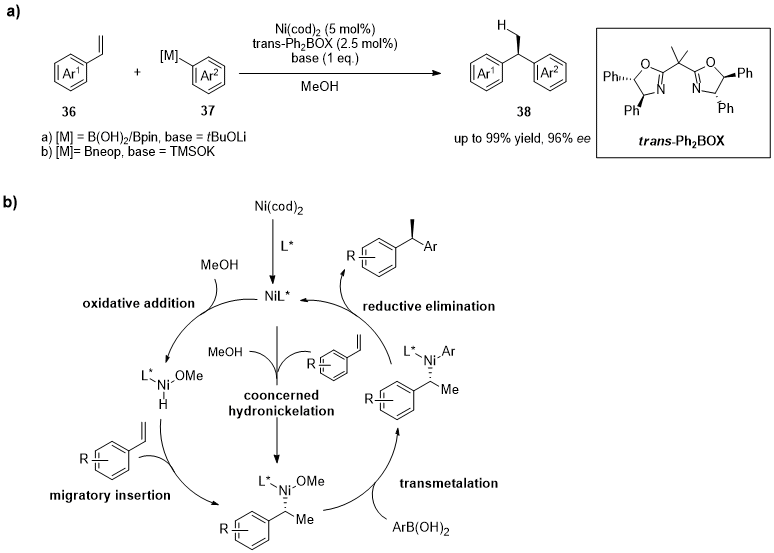
2. Nickel
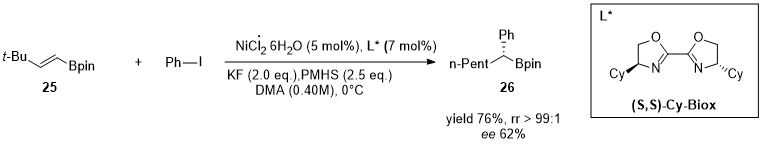
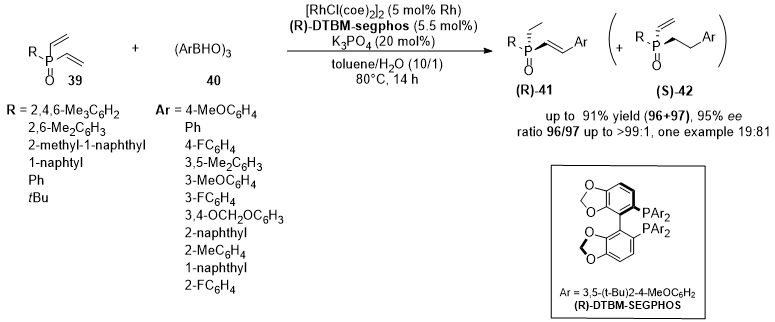
3. Rhodium
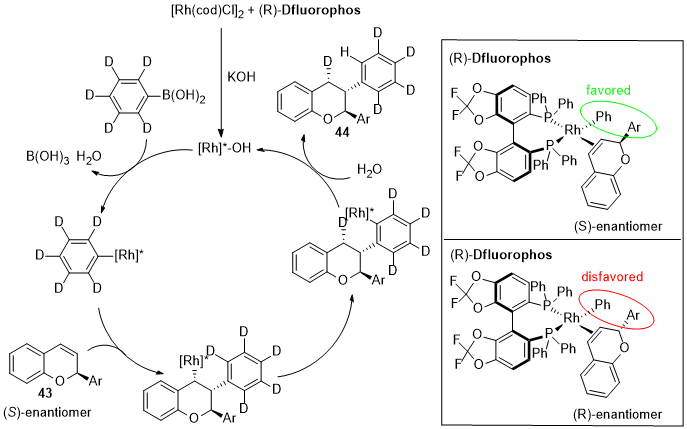
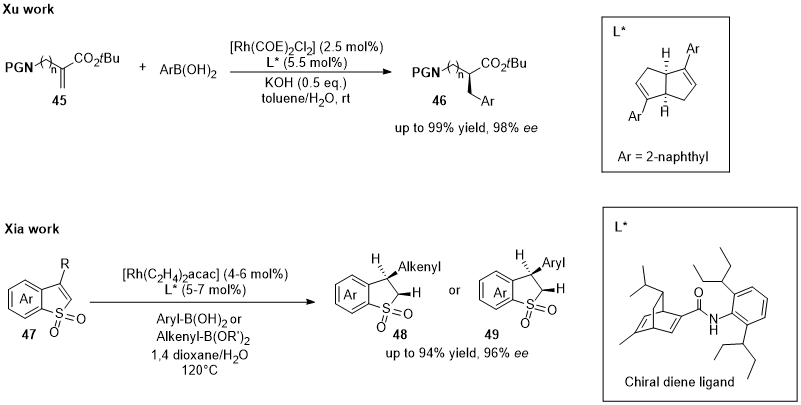
This entry is adapted from the peer-reviewed paper 10.3390/catal12101289
References
- Cacchi, S. The palladium-catalyzed hydroarylation and hydrovinylation of carbon-carbon multiple bonds: New perspectives in organic synthesis. Pure Appl. Chem. 1990, 62, 713–722.
- Cacchi, S.; Arcadi, A. Palladium-Catalyzed Conjugate Addition Type Reaction of Aryl Iodides with α,β-Unsaturated Ketones. J. Org. Chem. 1983, 48, 4236–4240.
- Fernández-Alvarez, F.-J.; Iglesias, M.; Oro, L.-A.; Passarelli, V. Bond Activation and Catalysis. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevie: Amsterdam, The Netherlands, 2013; pp. 399–432.
- Cacchi, S.; Fabrizi, G. Carbopalladation of Alkynes Followed by Trapping with Nucleophilic Reagents. In Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley Online: Hoboken, NJ, USA, 2002; pp. 1335–1359.
- Liao, L.; Sigman, M.-S. Palladium-Catalyzed Hydroarylation of 1,3-Dienes with Boronic Esters via Reductive Formation of π-Allyl Palladium Intermediates under Oxidative Conditions. J. Am. Chem. Soc. 2010, 132, 10209–10211.
- Podhajsky, S.-M.; Iwai, Y.; Cook-Sneathen, A.; Sigman, M.-S. Asymmetric palladium-catalyzed hydroarylation of styrenes and dienes. Tetrahedron 2011, 67, 4435–4441.
- Liu, S.; Zhou, J.-S. Desymmetrization of cyclic olefins via asymmetric Heck reaction and hydroarylation. Chem. Commun. 2013, 49, 11758–11760.
- Friis, S.-D.; Pirnot, M.-T.; Buchwald, S.-L. Asymmetric Hydroarylation of Vinylarenes Using a Synergistic Combination of CuH and Pd Catalysis. J. Am. Chem. Soc. 2016, 138, 8372–8375.
- Lu, Z.; Buchwald, S.-L. Enantioselective Preparation of Arenes with b-Stereogenic Centers: Confronting the 1,1-Disubstituted Olefin Problem Using CuH/Pd Cooperative Catalysis. Angew. Chem. Int. Ed. 2020, 59, 16128–16132.
- Schuppe, A.-W.; Borrajo-Calleja, G.-M.; Buchwald, S.-L. Enantioselective Olefin Hydrocyanation without Cyanide. J. Am. Chem. Soc. 2019, 141, 18668–18672.
- Chang, J.; Reiner, J.; Xie, J. Progress on the Chemistry of Dibenzocyclooctadiene Lignans. J. Chem. Rev. 2005, 105, 4581–4609.
- Lednicer, D. The Organic Chemistry of Drug Synthesis. In Organic Chem. Synthesis; Wiley: Hoboken, NJ, USA, 2007; Volume 7.
- Wang, R.; Liu, D.; Li, X.; Zhang, J.; Cui, D.; Wan, X. Synthesis and Stereospecific Polymerization of a Novel Bulky Styrene Derivative. Macromolecules 2016, 49, 2502–2510.
- Kong, W.; Wang, Q.; Zhu, J. Water as a Hydride Source in Palladium-Catalyzed Enantioselective Reductive Heck Reactions. Angew. Chem. Int. Ed. 2017, 56, 3987–3991.
- Zhang, Z.-M.; Xu, B.; Qian, Y.; Wu, L.; Wu, Y.; Zhou, L.; Liu, Y.; Zhang, J. Palladium-Catalyzed Enantioselective Reductive Heck Reactions:Convenient Access to 3,3-Disubstituted 2,3-Dihydrobenzofuran. Angew. Chem. Int. Ed. 2018, 130, 10530–10534.
- Oxtoby, L.-J.; Li, Z.-Q.; Tran, V.-T.; Erbay, T.-G.; Deng, R.; Liu, P.; Engle, K.-M. A Transient-Directing-Group Strategy Enables Enantioselective Reductive Heck Hydroarylation of Alkenes. Angew. Chem. Int. Ed. 2020, 132, 8970–8975.
- Zhang, Y.; Han, B.; Zhu, S. Rapid Access to Highly Functionalized Alkyl Boronates by NiH-Catalyzed Remote Hydroarylation of Boron-Containing Alkenes. Angew. Chem. Int. Ed. 2019, 131, 13998–14002.
- He, Y.; Liu, C.; Yu, L.; Zhu, S. Enantio- and Regioselective NiH-Catalyzed Reductive Hydroarylation of Vinylarenes with Aryl Iodides. Angew. Chem. Int. Ed. 2020, 59, 21530–21534.
- Xue, Y.; Chen, J.; Song, P.; He, Y.; Zhu, S. Nickel-Catalyzed Regiodivergent Reductive Hydroarylation of Styrenes. Synlett 2021, 32, 1647–1651.
- Zhang, Y.; Ma, J.; Chen, J.; Meng, L.; Liang, Y.; Zhu, S. A relay catalysis strategy for enantioselective nickel-catalyzed migratory hydroarylation forming chiral a-aryl alkylboronates. Chem 2021, 7, 3171–3188.
- He, Y.; Song, H.; Chen, J.; Zhu, S. NiH-catalyzed asymmetric hydroarylation of N-acyl enamines to chiral benzylamines. Nat. Commun. 2021, 12, 638.
- Cuesta-Galisteo, S.; Schçrgenhumer, J.; Wei, X.; Merino, E.; Nevado, C. Nickel-Catalyzed Asymmetric Synthesis of a-Arylbenzamides. Angew. Chem. Int. Ed. 2021, 133, 1629–1633.
- Chen, Y.-G.; Shuai, B.; Xu, X.-T.; Li, Y.-Q.; Yang, Q.-L.; Qiu, H.; Zhang, K.; Fang, P.; Mei, T.-S. Nickel-catalyzed Enantioselective Hydroarylation and Hydroalkenylation of Styrenes. J. Am. Chem. Soc. 2019, 141, 3395–3399.
- Lv, X.-Y.; Fan, C.; Xiao, L.-J.; Xie, J.-H.; Zhou, Q.-L. Ligand-Enabled Ni-Catalyzed Enantioselective Hydroarylation of Styrenes and 1,3-Dienes with Arylboronic Acids. CCS Chem. 2019, 1, 328–334.
- Marcum, J.-S.; Taylor, T.-R.; Meek, S.-J. Enantioselective Synthesis of Functionalized Arenes by Nickel-Catalyzed Site-Selective Hydroarylation of 1,3-Dienes with Aryl Boronates. Angew. Chem. Int. Ed. 2020, 59, 14070–14075.
- Tran, H.-N.; Burgett, R.-W.; Stanley, L.-M. Nickel-Catalyzed Asymmetric Hydroarylation of Vinylarenes: Direct Enantioselective Synthesis of Chiral 1,1-Diarylethanes. J. Org. Chem. 2021, 86, 3836–3849.
- Sakai, M.; Hayashi, H.; Miyaura, N. Rhodium-catalyzed conjugate addition of aryl-or 1-alkenylboronic acids to enones. Organometallics 1997, 16, 4229.
- Takaya, Y.; Ogasawara, M.; Hayashi, T.; Sakai, M.; Miyaura, N. Rhodium-catalyzed asymmetric 1, 4-addition of aryl-and alkenylboronic acids to enones. J. Am. Chem. Soc. 1998, 120, 5579–5580.
- Hayashi, T.; Yamasaki, K. Rhodium-catalyzed asymmetric 1, 4-addition and its related asymmetric reactions. Chem. Rev. 2003, 103, 2829–2844.
- Berthon, G.; Hayashi, T. Catalytic Asymmetric Conjugate Reactions; Córdova, A., Ed.; Wiley-VCH: Weinheim, Germany, 2010; Chapter 1; p. 1.
- So, C.M.; Kume, S.; Hayashi, T. Rhodium-Catalyzed Asymmetric Hydroarylation of 3-Pyrrolines Giving 3-Arylpyrrolidines: Protonation as a Key Step. J. Am. Chem. Soc. 2013, 135, 10990–10993.
- Wang, Z.; Hayashi, T. Rhodium-Catalyzed Enantioposition-Selective Hydroarylation of Divinylphosphine Oxides with Aryl Boroxines. Angew. Chem. Int. Ed. 2018, 130, 1718–1722.
- Yang, Q.; Wang, Y.; Luo, S.; Jun Wang, J. Kinetic Resolution and Dynamic Kinetic Resolution of Chromene by Rhodium-Catalyzed Asymmetric Hydroarylation. Angew. Chem. Int. Ed. 2019, 131, 5397–5401.
- Chen, J.P.; Li, Y.; Liu, C.; Wang, T.; Chung, L.W.; Xu, M.H. Water as a Direct Proton Source for Asymmetric Hydroarylation Catalyzed by a Rh(I)−Diene: Access to Nonproteinogenic β2/γ2/δ2-Amino Acid Derivatives. Org. Lett. 2021, 23, 571–577.
- Hu, F.; Jia, J.; Li, X.; Xia, Y. Enantioselective Hydroarylation or Hydroalkenylation of Benzothiophene 1,1-Dioxides with Organoboranes. Org. Lett. 2021, 23, 896–901.
