Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Edible mushrooms are considered an important next-generation healthy food source. Edible mushrooms are rich in proteins, dietary fiber, vitamins, minerals, and other bioactive components (alkaloids, lactones, polysaccharides, polyphenolic compounds, sesquiterpenes, sterols, and terpenoids).
- anticancer
- antidiabetic
- antioxidants
- phenolics
- poisonous mushroom
- biomolecules
1. Sustainable Production of Edible Mushrooms
More than 100 countries cultivate edible mushrooms commercially using different systems and on different scales. The global production of mushrooms increases annually at growth rate of about 7%. The current global consumption of mushrooms is around 12.74 million tonnes, and it is predicted that the global production of mushrooms will scale to 20.84 million tonnes by 2026 [1]. Mushrooms can be classified into some categories including (1) cultivated mushrooms (which can be grown commercially by farmers using various strategies to produce for sale at supermarkets; these include enoki and oyster mushrooms), (2) wild mushrooms (which can grow on the root systems of trees in forests and are harvested by mushroom hunters—lots of wild mushrooms are poisonous), (3) medicinal mushrooms (several mushrooms have medicinal benefits but might not always be pleasant to consume), and (4) poisonous mushrooms, which have toxic substances or toxins [2]. Based on the growing method of mushrooms, they can be classified into saprotrophic mushrooms (sapro = rot; troph = eating), which grow on dead matter; mycorrhizal mushrooms (mykes = fungus; rhiza = root), which have a symbiotic relationship (sym = together; biosis = way of living) with crops or trees; and parasitic mushrooms, which infect and depend on its host plant and then kill it [2].
Most cultivated mushrooms have a basic life cycle, which includes the following stages (1) sporulation (i.e., production spores not seeds), (2) spore germination and the mating of cells, (3) colonization to complete fruiting initiated, (4) the formation of primordia, and (5) after fruiting or mature of mushrooms, spores release again and the cycle repeats (Figure 1) [3]. The cultivation steps can be summarized in terms of seven stages, as presented in Figure 2 and described in [3]. The sustainable production of mushrooms is important because mushrooms are considered edible foods and are high in protein content. There are many poisonous mushrooms, which must be clear to consumers in order to avoid causing serious ecological and health problems [4]. The huge amount of wastes that result from mushroom cultivation needs to be sustainably recycled for in order to protect the environment and to produce the bioenergy as well. Therefore, edible mushrooms can be promoted as an important agri-business activity to address many environmental issues, especially the ecological degradation [1][5]. The cultivation of mushrooms can be used to manage farm wastes by recycling them, as well as to dispose of spent mushroom substrate “SMS” (i.e., wastes remaining after harvesting mushrooms). On the other hand, the cultivation of mushrooms should protect from many competitor microorganisms and promote hygiene, which are controlled through the environmental conditions as presented in Table 1 [6].

Figure 1. The industry of mushroom cultivation is considered an important industry, which needs certain steps to produce the edible mushrooms as presented in these photos. The first group of photos (1) includes mushroom fungi (Pleurotus ostreatus) culture, which should first be ready on the surface of agar plate beside the cultivation substrates, as well as tools for propagation of mushroom and poured media (heat treated at 95 °C, for 1 day). The inoculant and the millet spawn in a jar should be also prepared. The second and third group of photos (2,3) include the industrial preparing and filling of spawn in the factory, whereas the group of photos in (4) represent oyster mushroom production. These steps have been photographed from the factory of “Magyar Gomba Kertész Kft”, and all photos were taken by Gréta Törős, Debrecen University, Hungary.
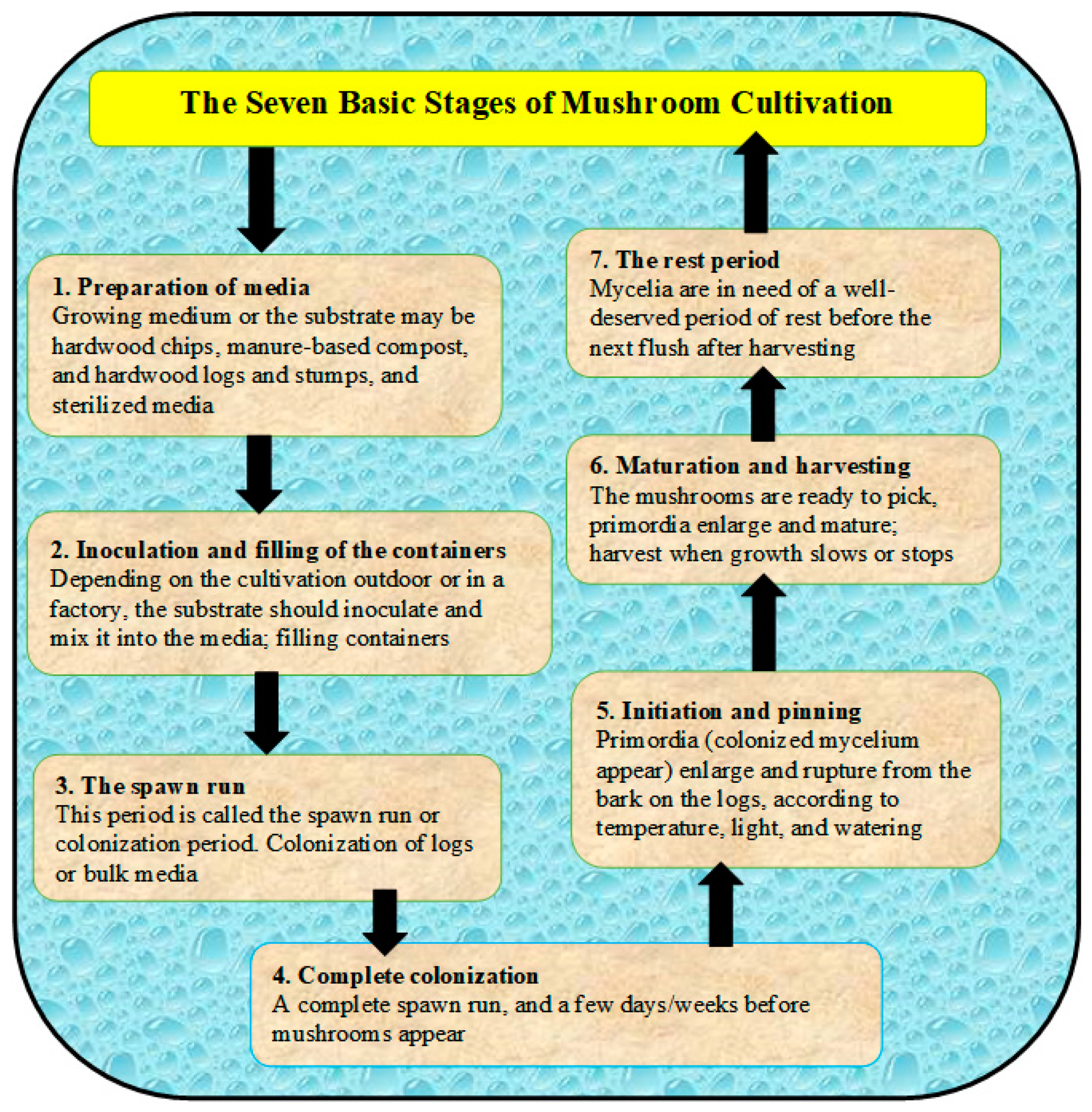
Figure 2. Different stages of mushroom cultivation starting from preparation of media till harvesting.
Table 1. The environmental conditions controlling the most commonly cultivated species as ideal values, but certain strains may exceed them.
| Environmental Conditions | Spawn Run | Primordia Formation | Fruitbody Development |
|---|---|---|---|
| Temperature | 21–27 °C | 10–21 °C | 10–24 °C |
| Relative Humidity | 85–95% | 95–100% | 80–90% |
| Light | 50–100 lux | 1000–1500 lux | 1000–1500 lux |
| CO2 level | 5000–20,000 ppm; 1 FAE/h | 500–2000 ppm; 4–8 FAE/h | <1000–2000 ppm; 4–8 FAE/h |
| Duration | 2–8 weeks | 3–12 days | 5–8 days |
Source: [6] Fresh Air Exchange (FAE).
2. Bioactive Ingredients of Edible Mushrooms
Edible mushrooms contain various bioactive ingredients such as proteins, polysaccharides, polyunsaturated fatty acids (PUFA), dietary fibers, amino acids, vitamins, and minerals (Table 2). They have essential health effects, such as antioxidant, antimicrobial, immune-stimulatory, and anticancer, cholesterol-lowering properties (Figure 3) [7][8][9][10]. Several significant components and secondary metabolites dominate their biological activity. Lectins are carbohydrate-binding proteins that can be found in many types of edible mushrooms such as Phaseolus vulgaris, Agaricus campestris, Agaricus bisporus, Grifola frondosa, Boletus satanus, Flammulina velutipes, Tricholoma mongolicum, Ganoderma lucidum, and Volvariella volvacea. Lectins have been shown to increase insulin secretion, activate the immune system, and have anticancer effects [11]. Lectins can also play essential roles in physiological processes such as dormancy, growth, morphogenesis, morphological changes, and molecular recognition in the early stages of mycorrhization. Lectins isolated from the edible mushroom Clitocybe nebularis exhibit immunostimulatory effects on the most potent antigen-presenting cells, dendritic cells [12].
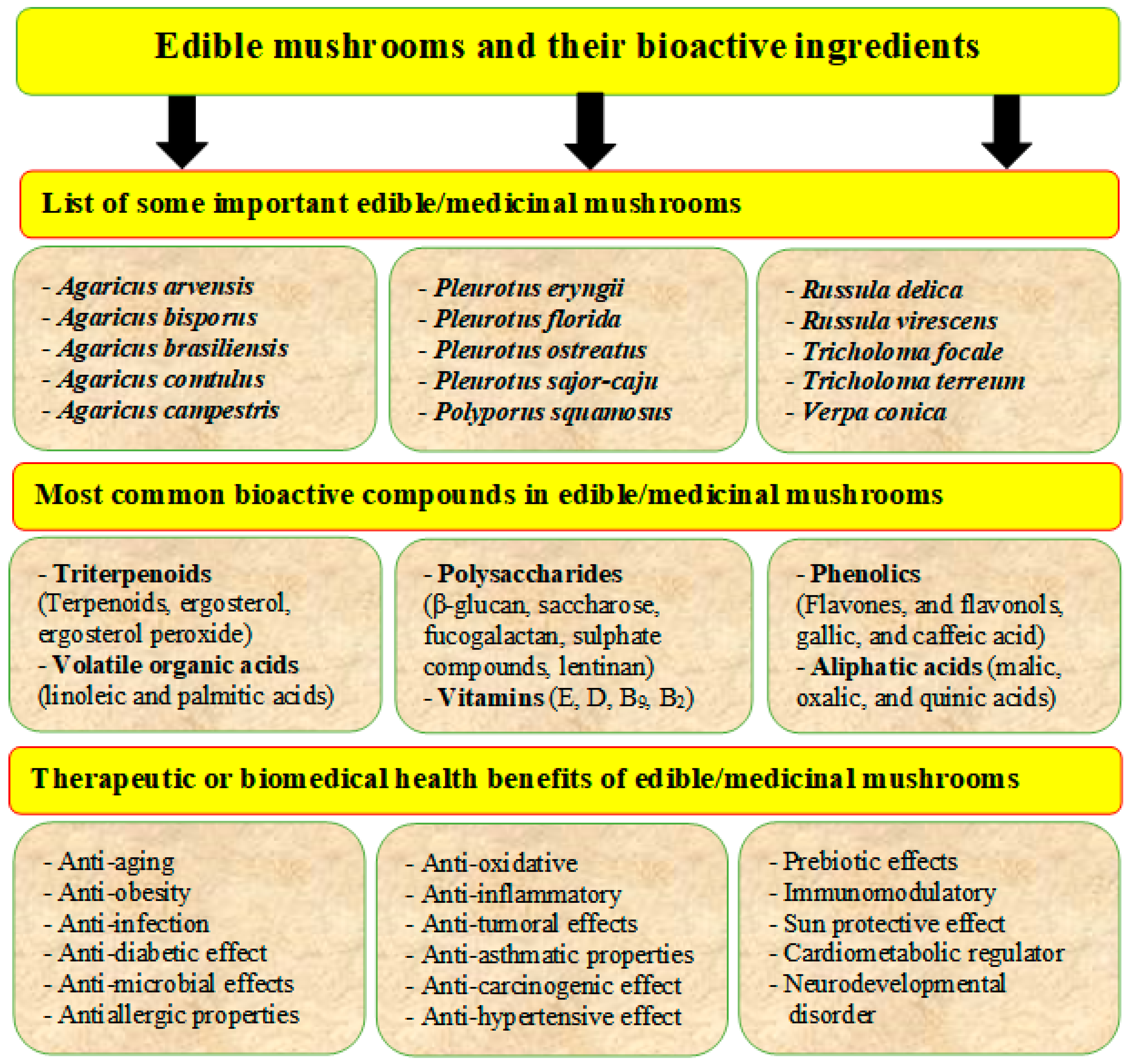
Figure 3. Edible mushrooms are an important source of human food and have several bioactive ingredients (phenols, lectins, terpenoids, flavonoids, etc.) and bio-medicinal activities such as anticancer, antidiabetic, antiaging, antiviral, antioxidant, and anti-inflammatory.
Table 2. A list of some phenolic compounds, polysaccharides, proteins, and triterpenoids found in selected edible mushrooms species.
| Edible Mushrooms | Phenolics | Polysaccharides | Proteins | Triterpenoids | Refs. |
|---|---|---|---|---|---|
| Agaricus bisporus | Gallocatechin | Heteropoly-saccharide ABP | Protein type FIIb-1 | Ergosterol | [13][14][15][16] |
| Boletus edulis | Gallic acid | Polysaccharides (BEBP-1) | β-Trefoil lectin | Boledulins A-C | [13][17][18][19] |
| Cordyceps aegerita | Proto-catechuic acid | Fucogalactan | Ageritin | Bovistols A-C | [20][21][22][23] |
| Coprinus comatus | Flavones, and flavonols | Modified polysaccharide | Laccases | Terpenoids | [24][25][26] |
| Lactarius deliciosus | Syringic acid, vanillic acid | Polysaccharide (LDG-M) | Laccase | Azulene-type sesquiterpene | [27][28][29][30] |
| Pleurotus ostreatus | Caffeic acid, and ferulic acid | Mycelium polysaccharides | Concanavalin A | Ergosterol | [31] |
| Pleurotus eryngii | Cinnamic acid | PEPE-A1 and PEPE-A2 | Laccase | Ergosterol | [32][33] |
| Pleurotus cornucopiae | Gallic acid | β-glucan | Oligopeptides | Ergostane-type sterols | [34][35][36][37] |
| Macrolepiota procera | Proto-catechuic acid | Polysaccharides | β-Trefoil lectin | Lanostane triterpenoids | [18][38][39][40] |
| Russula virescens | Quercetin | SRVPs | Laccase | ---------- | [41][42][43] |
| Tuber melanosporum | Flavonoids, phenols | Exo-poly-saccharides (TP1) | ------------- | Ergosterol | [16][35][36][37][38][39][40][41][42][43][44] |
Abbreviations: FIP (Immunomodulatory proteins); PEPE (Pleurotus eryngii purified polysaccharides); PSK (Polysaccharide K); PSP (Polysaccharide peptide); RVP (Russula virescens polysaccharide); LDG m (Lactarius deliciosus polysaccharides); BEPF (crude polysaccharides isolated from B. edulis).
Glucans are one of the unique ingredients in mushrooms that have immune-stimulatory, anticancer, anti-inflammatory, and antioxidant effects. They can be found in different types of edible mushrooms such as Jelly ears (Auricularia auricular), Reishi (Ganoderma lucidum), Shiitake (Lentinula edodes), and Oyster (Pleurotus ostreatus) [45]. For example, beta-glucan, isolated from Pleurotus pulmonarius, has potent anti-inflammatory and antinociceptive properties that inhibit pro-inflammatory cytokines [46]. Glucans isolated from Pleurotus pulmonarius also suppressed colon carcinogenesis associated with colitis by regulating cell proliferation, inducing apoptosis, and suppressing inflammation [47]. Beta-glucan is a high-molecular-weight polysaccharide of glucose bound by glycosidic bonds.
Phenolic compounds are secondary metabolites of edible mushrooms. Polyphenols have been extensively studied and shown to be effective against a variety of health complications. Phenolic acids such as p-hydroxybenzoic, cinnamic, gallic, salicylic, p-coumaric acids, syringic acids, caffeic, ferulic, chlorogenic, and flavonoid can be found in mushrooms [48]. Gallic, caffeic, and p-coumaric acids are the main phenolic groups and play an essential role in the biological activity of mushrooms [49]. Phenolic compounds have high antioxidant activity. Polyphenols from edible mushrooms such as Meripilus giganteus, Agaricus hydnum, and Rufescens silvaticus have high antioxidant capacity [50]. Phenolic compounds have also shown anticancer activity against kidney cancer cell lines and human ovarian cancer cell lines [51]. Flavonoid compounds, including myricetin, rutin, naringenin, quercetin, morin, and hesperetin, are included in the polyphenol content, and they exhibit antiproliferative effect [52].
Terpenoids such as monoterpenoids, sesquiterpenoids, diterpenoids, and triterpenoids, are essential components in mushrooms. They have been shown to have antimicrobial, anticancer, anticholinesterase, anti-inflammatory, antimalarial, and antioxidant properties [21]. Currently, about 285 types of terpenoids have been discovered in mushrooms and have medicinal properties. For example, ganoderic acids are a lanastanoid type triterpenoid and have been isolated from Ganoderma amboinense, Ganoderma lucidum, etc. Ergosterol, the principal sterol in most edible mushrooms, is a valuable dietary precursor of vitamin D2 and a natural antioxidant [53]. Phytosterols, such as ergosterols and ergosterol peroxide, have been shown to be more potent than the nonsteroidal anti-inflammatory drug indomethacin, as shown by their 50% inhibitory effect. High levels of ergosterols can be found in Agaricus bisporus, Lentinus edodes, Grifola frondose, Pleurotus ostreatus, Agaricus bisporus, and Agaricus bisporus [7].
3. Nutritional Values and Health Benefits of Edible Mushrooms
As a crucial source of food for humans for thousands of years, the medicinal and organoleptic properties provided by the chemical composition and nutritional value of edible mushrooms have been reported by several researchers (Figure 4). The consumption of edible and medicinal mushrooms in Eastern and Western countries has gradually increased in recent decades [54]. Agaricus bisporus, Lentinus edodes, and Pleurotus spp. are presently the most common cultivated edible mushrooms, with China as the largest producer of these mushrooms in the world [55]. Edible mushrooms are known for their high contents of carbohydrates, protein, and crude fibers, as well as different bioactive compounds, which provide both nutritional and health benefits for humans [55][56]. The relative content of these nutritional components differs by species and between countries, as reported in Table 3.

Figure 4. Photos of some common edible mushrooms and their scientific names. Sources: by frankenstoen from Portland, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=7304024; CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=191739; by Dan Molter (shroomydan), CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=9822537; by TOMMES-WIKI, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=27753655; by Strobilomyces, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=179148; by Archenzo, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=24474; by voir ci-dessous, CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=3330811; by Mыць Дeниc, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1334049, accessed on 16 January 2020.
Table 3. General nutritional values (based on dry weight) of some common edible mushrooms from different sources.
| Mushroom Species Used in the Study | Moisture (%) | Total Protein (%) | Total Carbo-Hydrate (%) | Crude Fiber (%) | Ash (%) | Total Phenols (%) | Refs. |
|---|---|---|---|---|---|---|---|
| I. Studied many species | |||||||
| Agaricus bisporus, Agrocybe cylindracea, Boletus loyo, Cortinarius lebre, Cyttaria espinosae | 86–96 | 8.56–23.88 | 62.97–83.65 | 7–15 | 5–13 | 0.75–4.72 | [55] |
| Apioperdon pyriforme, Helvella elastica, Morchella conica and Rhizopogon luteolus) | - | 11.5–24.5 | - | 2.6–4.8 | 9.5–14.7 | 5.0–12.3 | [57] |
| Auricularia auricula-judae, A. polytricha, Lactifluus piperatus, Laetiporus sulphureus | 49–88 | 19–56 | 7–18 | 5–11 | 3–8 | 7.3–10.2 | [58] |
| Auricularia auricular, Ganoderma lucidum, Pleurotus citrinopleatus, P. djamor, P. eryngii, P. ostreatus, and P. ostreatus | 4–11 | 20–45 | 11–61 | 5–40 | 6–10 | 1–8 | [59] |
| Agaricus bisporus, Pleurotus ostreatus, Lentinula edodes | 88–92 | 1.7–2.11 | 3–9 | 20–37 | 0.8–1.15 | 1.1–1.5 | [60] |
| Tricholoma, Shiitake mushroom, Pleurotus eryngii, Dictyophora indusiata | 6.9–15.5 | 8.5–36.9 | 0.5–37.3 | 14.4–70.2 | 1.3–10.1 | - | [61] |
| Agaricus bisporus, Boletus edulis, Cantharellus cibarius, Lactarius deliciosus, Leccinum rufom | 82.6–91 | 1.5–3.6 | 3.2–8.3 | - | - | - | [62] |
| Astraeus odoratus, Craterellus aureus, Lentinus edodes, Phaeogyroporus portentosus | - | 13.1–32.8 | 2.79–44.3 | 77.1 | - | - | [63] |
| Agaricus bisporus, Boletus edulis, Calocybe indica, C. gambosa, Grifola frondosa, Flammulina velutipes | - | 18.1–62.8 | 31.1–70.6 | 7.81–32.3 | 3.5–19.7 | - | [56] |
| Pleurotus sajor-caju; Calocybe indica | 87–89 | 1.74–3.4 | 3.37–3.33 | - | 1.2–1.3 | - | [64] |
| II. Studied only one species | |||||||
| Agaricus bisporus | 81.79 | 29.29 | 20.57 | 24.56 | 7.12 | - | [65] |
| Flammulina velutipes | - | 18.42 | 56.37 | 7.81 | 6.33 | - | [66] |
| Grifola frondosa | 83.06 | 21.1 | 58.8 | 10.1 | 7.0 | - | [67] |
| Hericium erinaceus | 95.69 | 23.3 | 57.0 | 7.8 | 9.4 | - | [67] |
| Lactarius deliciosus | 92 | 17.19 | 66.61 | 31.81 | 8.62 | 4.5–13.6 | [68] |
| Lyophyllum decastes | - | 18.31 | 34.36 | 29.02 | 14.20 | - | [69] |
| Pleurotus florida | 87.05 | 34.56 | 31.59 | 11.41 | 7.40 | - | [70] |
| Pleurotus ostreatus | 85.55 | 30.92 | 31.40 | 12.10 | 7.05 | - | [70] |
| Russula delica | - | 26.25 | 34.88 | 15.42 | 17.92 | - | [69] |
| Tremella fuciformis | 91.73 | 4.6 | 94.8 | 1.4 | 0.4 | - | [71] |
| Tricholoma giganteum | - | 16.1 | 70.1 | 4.5 | 5.0 | - | [72] |
| Volvariella volvacea | - | 30.1 | 50.9 | 11.9 | 12.6 | - | [72] |
The main nutritional compounds that edible mushrooms are rich in include proteins, carbohydrates, crude fiber, total phenolic compounds, and vitamins and many minerals. Several studies have discussed the nutritional value of edible mushrooms from different locations such as Argentina [60], Bangladesh [59], China [60][61], Chile [55], India [57][58], and Turkey [73][74]. Many reports have also focused on one or more edible mushrooms such as Agaricus bisporus [75][76]; Lactarius deliciosus [68]; Pleurotus citrinopileatus [77]; Helvella leucopus and Morchella pulchella [73]; Pleurotus sajor-caju and Calocybe indica [64]; Agaricus bisporus, Cantharellus cibarius, and Lentinula edodes [50]; some studies have focused on several species, e.g., Altaf et al. [57], Das et al. [56], and Taskın et al. [51]. Several myco-chemical structures and compositions can be found in edible mushrooms, including phenolic compounds, fatty acids, terpenoids, lipids, polysaccharides, and proteins, which are important compounds for human health. Saini et al. [78] reported the biologically active nutraceutical compounds present in edible mushrooms, including ergosterol, proteins, and fatty acids content (388 mg/100 g DW, 4.5–37.4%, and 1.75–15.5%, respectively).
The biofortification approach can be defined as a method by which pollutants and/or biodegradation from the environment can be removed or blocked from microorganisms such as bacteria, fungi, or algae. It is considered a sustainable agricultural strategy and a cost-effective tool to increase the bioavailability or content of essential nutrients in the edible parts of cultivated plants and reduce malnutrition [79]. Some edible mushrooms have been biofortified with nutrients, particularly selenium [80], which is applied to produce Se enrichment and increase bioactive metabolites of Pleurotus ostreatus [81], Cordyceps militaris [82], Hericium erinaceus [83], and Ganoderma lucidum [84]. Nano-biofortification is also an important approach that recently has been confirmed for using nano-selenium as anti-COVID-19 nanoparticles [85][86].
This entry is adapted from the peer-reviewed paper 10.3390/su14094941
References
- Shirur, M.; Barh, A.; Annepu, S.K. Sustainable Production of Edible and Medicinal Mushrooms: Implications on Mushroom Consumption. In Climate Change and Resilient Food Systems; Hebsale Mallappa, V.K., Shirur, M., Eds.; Springer: Singapore, 2021; pp. 315–346. ISBN 978-981-334-537-9.
- Korman, R. Growing Mushrooms: The Complete Grower’s Guide to Becoming a Mushroom Expert and Starting Cultivation at Home; Amazon Digital Services LLC: Seattle, WA, USA, 2020; ISBN 978-1-65911-727-1.
- Cotter, T. Organic Mushroom Farming and Mycoremediation: Simple to Advanced and Experimental Techniques for Indoor and Outdoor Cultivation; Chelsea Green Publishing: White River Junction, VT, USA, 2015.
- Shirur, M. Entrepreneurial Behaviour and Socio Economic Analysis of Mushroom Growers in Karnataka. Indian J. Agric. Sci. 2017, 6, 840–845.
- El-Ramady, H.; Abdalla, N.; Fawzy, Z.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability 2022, 14, 3667.
- Arevalo, W. DIY Mushroom Cultivation: Growing Mushrooms at Home for Food, Medicine, and Soil. New Society Publishers, Gabriola Island, BC V0R 1X0, Canada. Available online: https://www.amazon.com/DIY-Mushroom-Cultivation-Mushrooms-Homesteader/dp/0865718954 (accessed on 17 March 2022).
- Nowak, R.; Nowacka-Jechalke, N.; Pietrzak, W.; Gawlik-Dziki, U. A New Look at Edible and Medicinal Mushrooms as a Source of Ergosterol and Ergosterol Peroxide-UHPLC-MS/MS Analysis. Food Chem. 2022, 369, 130927.
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Cruz-Martins, N.; Dhanjal, D.S.; Singh, R.; Chopra, C.; Verma, R.; Abd-Elsalam, K.A.; et al. Potential Usage of Edible Mushrooms and Their Residues to Retrieve Valuable Supplies for Industrial Applications. JoF 2021, 7, 427.
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy Function and High Valued Utilization of Edible Fungi. Food Sci. Hum. Wellness 2021, 10, 408–420.
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Update on Potential of Edible Mushrooms: High-value Compounds, Extraction Strategies and Bioactive Properties. Int. J. Food Sci. Technol. 2022, 57, 1378–1385.
- El-Maradny, Y.A.; El-Fakharany, E.M.; Abu-Serie, M.M.; Hashish, M.H.; Selim, H.S. Lectins Purified from Medicinal and Edible Mushrooms: Insights into Their Antiviral Activity against Pathogenic Viruses. Int. J. Biol. Macromol. 2021, 179, 239–258.
- Pohleven, J.; Brzin, J.; Vrabec, L.; Leonardi, A.; Čokl, A.; Štrukelj, B.; Kos, J.; Sabotič, J. Basidiomycete Clitocybe nebularis Is Rich in Lectins with Insecticidal Activities. Appl. Microbiol. Biotechnol. 2011, 91, 1141–1148.
- Fogarasi, M.; Socaci, S.; Dulf, F.; Diaconeasa, Z.; Fărcaș, A.; Tofană, M.; Semeniuc, C. Bioactive Compounds and Volatile Profiles of Five Transylvanian Wild Edible Mushrooms. Molecules 2018, 23, 3272.
- Alshammaa, D.A.S. Phytochemical Investigation and Quantitative Comparison of Ergosterol Between Agaricus bisporus and Pleurotus ostreatus by HPLC and GC-MS Methods. Int. J. Pharm. Sci. Rev. Res. 2017, 44, 215–220.
- Verma, N.K.; Singh, A.P.; Singh, V.K. Agaricus bisporus (Fungi) Chemical Constituents and Pharmacological Activities—A Review. Asian J. Phytomedicine Clin. Res. 2019, 7, 82–87.
- Liu, G.; Ye, J.; Li, W.; Zhang, J.; Wang, Q.; Zhu, X.; Miao, J.; Huang, Y.; Chen, Y.; Cao, Y. Extraction, Structural Characterization, and Immunobiological Activity of ABP Ia Polysaccharide from Agaricus bisporus. Int. J. Biol. Macromol. 2020, 162, 975–984.
- Feng, T.; Li, Z.-H.; Dong, Z.-J.; Su, J.; Li, Y.; Liu, J.-K. Non-Isoprenoid Botryane Sesquiterpenoids from Basidiomycete Boletus edulis and Their Cytotoxic Activity. Nat. Prod. Bioprospect. 2011, 1, 29–32.
- Žurga, S.; Nanut, M.P.; Kos, J.; Sabotič, J. Fungal Lectin MpL Enables Entry of Protein Drugs into Cancer Cells and Their Subcellular Targeting. Oncotarget 2017, 8, 26896–26910.
- Luo, A.; Luo, A.; Huang, J.; Fan, Y. Purification, Characterization and Antioxidant Activities in Vitro and in Vivo of the Polysaccharides from Boletus edulis Bull. Molecules 2012, 17, 8079–8090.
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P.; Kozak, L. Phenolic and Flavonoid Content in Hericium erinaceus, Ganoderma lucidum, and Agrocybe aegerita under Selenium Addition. Acta Aliment. 2016, 45, 300–308.
- Surup, F.; Hennicke, F.; Sella, N.; Stroot, M.; Bernecker, S.; Pfütze, S.; Stadler, M.; Rühl, M. New Terpenoids from the Fermentation Broth of the Edible Mushroom Cyclocybe aegerita. Beilstein J. Org. Chem. 2019, 15, 1000–1007.
- Citores, L.; Ragucci, S.; Ferreras, J.M.; Maro, A.D.; Iglesias, R.; Citores, L.; Ferreras, J.M.; Iglesias, R. Ageritin, a Ribotoxin from Poplar Mushroom (Agrocybe aegerita) with Defensive and Antiproliferative Activities. ACS Chem. Biol. 2019, 14, 1319–1327.
- Motoshima, R.A.; Rosa, T.D.F.; Mendes, L.D.C.; da Silva, E.V.; Viana, S.R.; do Amaral, B.S.; de Souza, D.H.; Lião, L.M.; da Silva, M.D.L.C.; de Sousa, L.R.; et al. Inhibition of Leishmania Amazonensis Arginase by Fucogalactan Isolated from Agrocybe aegerita Mushroom. Carbohydr. Polym. 2018, 201, 532–538.
- Nowakowski, P.; Naliwajko, S.K.; Markiewicz-Żukowska, R.; Borawska, M.H.; Socha, K. The Two Faces of Coprinus comatus—Functional Properties and Potential Hazards. Phytother. Res. 2020, 34, 2932–2944.
- Dulay, M.R.; Sanguesa, K.B.; Ablaza, J.; Joson, A.J.M.; Peria, J.N.T.; Quejada, C.S.; Basa, J.O.; Castro, M. Bioactive Myco-Nutrients of Aseptically Cultured Fruiting Bodies of coprinus comatus (o.f. Müll.) Pers. On Rice Bran-Enriched Ruminants’ Dung. Available online: https://www.semanticscholar.org/paper/BIOACTIVE-MYCO-NUTRIENTS-OF-ASEPTICALLY-CULTURED-OF-Dulay-Sanguesa/6ab75659b7e5c608804664d0bc0d3618a33798ad (accessed on 7 January 2022).
- Zhao, H.; Zhang, J.; Liu, X.; Yang, Q.; Dong, Y.; Jia, L. The Antioxidant Activities of Alkalic-Extractable Polysaccharides from Coprinus comatus on Alcohol-Induced Liver Injury in Mice. Sci. Rep. 2019, 8, 11695.
- Kalogeropoulos, N.; Yanni, A.E.; Koutrotsios, G.; Aloupi, M. Bioactive Microconstituents and Antioxidant Properties of Wild Edible Mushrooms from the Island of Lesvos, Greece. Food Chem. Toxicol. 2013, 55, 378–385.
- Feussi Tala, M.; Qin, J.; Ndongo, J.T.; Laatsch, H. New Azulene-Type Sesquiterpenoids from the Fruiting Bodies of Lactarius deliciosus. Nat. Prod. Bioprospect. 2017, 7, 269–273.
- Khaund, P.; Joshi, S.R. Enzymatic Profiling of Wild Edible Mushrooms Consumed by the Ethnic Tribes of India. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 263–271.
- Su, S.; Ding, X.; Fu, L.; Hou, Y. Structural Characterization and Immune Regulation of a Novel Polysaccharide from Maerkang Lactarius deliciosus Gray. Int. J. Mol. Med. 2019, 44, 713–724.
- Sarma, D.; Saha, A.K.; Datta, B.K. Bioactive Compounds with Special References to Anticancer Property of Oyster Mushroom. J. Pharmacogn. Phytochem. 2018, 7, 2694–2698.
- Souilem, F.; Fernandes, Â.; Calhelha, R.C.; Barreira, J.C.M.; Barros, L.; Skhiri, F.; Martins, A.; Ferreira, I.C.F.R. Wild Mushrooms and Their Mycelia as Sources of Bioactive Compounds: Antioxidant, Anti-Inflammatory and Cytotoxic Properties. Food Chem. 2017, 230, 40–48.
- Fu, Z.; Liu, Y.; Qiang, Z. A Potent Pharmacological Mushroom: Pleurotus eryngii. Fungal Genom. Biol. 2016, 6, 1–5.
- Alam, N.; Yoon, K.; Shin, P.; Cheong, J.-C.; Yoo, Y.; Lee, T.-S. Antioxidant, Phenolic Compounds Concentration, Xanthine Oxidase and Tyrosinase Inhibitory Activities of Pleurotus cornucopiae. Aust. J. Basic Appl. Sci. 2011, 5, 229–239.
- Lee, S.R.; Lee, D.; Lee, H.-J.; Noh, H.J.; Jung, K.; Kang, K.S.; Kim, K.H. Renoprotective Chemical Constituents from an Edible Mushroom, Pleurotus cornucopiae in Cisplatin-Induced Nephrotoxicity. Bioorg. Chem. 2017, 71, 67–73.
- Golak-Siwulska, I.; Kałużewicz, A.; Spiżewski, T.; Siwulski, M.; Sobieralski, K. Bioactive Compounds and Medicinal Properties of Oyster Mushrooms (Pleurotus sp.). Folia Hortic. 2018, 30, 191–201.
- Minato, K.; Ohara, A.; Mizuno, M. A Proinflammatory Effect of the β -Glucan from Pleurotus cornucopiae Mushroom on Macrophage Action. Mediat. Inflamm. 2017, 2017, 8402405.
- Li, X.; Zhang, X.; Ye, L.; Kang, Z.; Jia, D.; Yang, L.; Zhang, B. LC-MS-Based Metabolomic Approach Revealed the Significantly Different Metabolic Profiles of Five Commercial Truffle Species. Front. Microbiol. 2019, 10, 2227.
- Chen, H.-P.; Zhao, Z.-Z.; Li, Z.-H.; Huang, Y.; Zhang, S.-B.; Tang, Y.; Yao, J.-N.; Chen, L.; Isaka, M.; Feng, T.; et al. Anti-Proliferative and Anti-Inflammatory Lanostane Triterpenoids from the Polish Edible Mushroom Macrolepiota procera. J. Agric. Food Chem. 2018, 66, 3146–3154.
- Nowak, R.; Nowacka-Jechalke, N.; Juda, M.; Malm, A. The Preliminary Study of Prebiotic Potential of Polish Wild Mushroom Polysaccharides: The Stimulation Effect on Lactobacillus Strains Growth. Eur. J. Nutr. 2018, 57, 1511–1521.
- Butkhup, L.; Samappito, W.; Jorjong, S. Evaluation of Bioactivities and Phenolic Contents of Wild Edible Mushrooms from Northeastern Thailand. Food Sci. Biotechnol. 2018, 27, 193–202.
- Zhu, M.-J.; Du, F.; Zhang, G.-Q.; Wang, H.-X.; Ng, T.-B. Purification a Laccase Exhibiting Dye Decolorizing Ability from an Edible Mushroom Russula virescens. Int. Biodeterior. Biodegrad. 2013, 82, 33–39.
- Li, H.; Wang, X.; Xiong, Q.; Yu, Y.; Peng, L. Sulfated Modification, Characterization, and Potential Bioactivities of Polysaccharide from the Fruiting Bodies of Russula virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447.
- Tejedor-Calvo, E.; Morales, D.; Marco, P.; Sánchez, S.; Garcia-Barreda, S.; Smiderle, F.R.; Iacomini, M.; Villalva, M.; Santoyo, S.; Soler-Rivas, C. Screening of Bioactive Compounds in Truffles and Evaluation of Pressurized Liquid Extractions (PLE) to Obtain Fractions with Biological Activities. Food Res. Int. 2020, 132, 109054.
- Ganeshpurkar, A.; Pardhi, P.; Bhadoriya, S.S.; Jain, N.; Rai, G.; Jain, A.P. Antioxidant Potential of White Oyster Culinary-Medicinal Mushroom, Pleurotus florida (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 491–498.
- Murphy, E.J.; Rezoagli, E.; Pogue, R.; Simonassi-Paiva, B.; Abidin, I.I.Z.; Fehrenbach, G.W.; O’Neil, E.; Major, I.; Laffey, J.G.; Rowan, N. Immunomodulatory Activity of β-Glucan Polysaccharides Isolated from Different Species of Mushroom—A Potential Treatment for Inflammatory Lung Conditions. Sci. Total Environ. 2022, 809, 152177.
- Lavi, I.; Nimri, L.; Levinson, D.; Peri, I.; Hadar, Y.; Schwartz, B. Glucans from the Edible Mushroom Pleurotus pulmonarius Inhibit Colitis-Associated Colon Carcinogenesis in Mice. J. Gastroenterol. 2012, 47, 504–518.
- Bahadori, M.B.; Sarikurkcu, C.; Yalcin, O.U.; Cengiz, M.; Gungor, H. Metal Concentration, Phenolics Profiling, and Antioxidant Activity of Two Wild Edible Melanoleuca Mushrooms (M. cognata and M. stridula). Microchem. J. 2019, 150, 104172.
- Taofiq, O.; Calhelha, R.C.; Heleno, S.; Barros, L.; Martins, A.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. The Contribution of Phenolic Acids to the Anti-Inflammatory Activity of Mushrooms: Screening in Phenolic Extracts, Individual Parent Molecules and Synthesized Glucuronated and Methylated Derivatives. Food Res. Int. 2015, 76, 821–827.
- Kała, K.; Krakowska, A.; Szewczyk, A.; Ostachowicz, B.; Szczurek, K.; Fijałkowska, A.; Muszyńska, B. Determining the Amount of Potentially Bioavailable Phenolic Compounds and Bioelements in Edible Mushroom Mycelia of Agaricus bisporus, Cantharellus cibarius, and Lentinula edodes. Food Chem. 2021, 352, 129456.
- Taşkın, H.; Süfer, Ö.; Attar, Ş.H.; Bozok, F.; Baktemur, G.; Büyükalaca, S.; Kafkas, N.E. Total Phenolics, Antioxidant Activities and Fatty Acid Profiles of Six Morchella Species. J. Food Sci. Technol. 2021, 58, 692–700.
- Saltarelli, R.; Palma, F.; Gioacchini, A.M.; Calcabrini, C.; Mancini, U.; De Bellis, R.; Stocchi, V.; Potenza, L. Phytochemical Composition, Antioxidant and Antiproliferative Activities and Effects on Nuclear DNA of Ethanolic Extract from an Italian Mycelial Isolate of Ganoderma lucidum. J. Ethnopharmacol. 2019, 231, 464–473.
- Papoutsis, K.; Grasso, S.; Menon, A.; Brunton, N.P.; Lyng, J.G.; Jacquier, J.-C.; Bhuyan, D.J. Recovery of Ergosterol and Vitamin D2 from Mushroom Waste-Potential Valorization by Food and Pharmaceutical Industries. Trends Food Sci. Technol. 2020, 99, 351–366.
- Lu, H.; Lou, H.; Hu, J.; Liu, Z.; Chen, Q. Macrofungi: A Review of Cultivation Strategies, Bioactivity, and Application of Mushrooms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2333–2356.
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional Value and Biological Properties of Chilean Wild and Commercial Edible Mushrooms. Food Chem. 2021, 356, 129651.
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible Mushrooms as Functional Ingredients for Development of Healthier and More Sustainable Muscle Foods: A Flexitarian Approach. Molecules 2021, 26, 2463.
- Altaf, U.; Lalotra, P.; Sharma, Y.P. Nutritional and Mineral Composition of Four Wild Edible Mushrooms from Jammu and Kashmir, India. Indian Phytopathol. 2020, 73, 313–320.
- Ao, T.; Deb, C.R. Nutritional and Antioxidant Potential of Some Wild Edible Mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089.
- Sifat, N.; Lovely, F.; Zihad, S.M.N.K.; Hossain, M.G.; Shilpi, J.A.; Grice, I.D.; Mubarak, M.S.; Uddin, S.J. Investigation of the Nutritional Value and Antioxidant Activities of Common Bangladeshi Edible Mushrooms. Clin. Phytosci. 2020, 6, 88.
- Di Anibal, C.; Farenzena, S.; Rodríguez, M.S.; Albertengo, L. Chemical Composition and Nutritional Value of Argentine Commercial Edible Mushrooms. J. Verbr. Lebensm. 2015, 10, 155–164.
- Yu, Q.; Guo, M.; Zhang, B.; Wu, H.; Zhang, Y.; Zhang, L. Analysis of Nutritional Composition in 23 Kinds of Edible Fungi. J. Food Qual. 2020, 2020, 8821315.
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and Algae as Sources of Medicinal and Other Biologically Active Compounds: A Review. Nutrients 2021, 13, 3178.
- Wunjuntuk, K.; Ahmad, M.; Techakriengkrai, T.; Chunhom, R.; Jaraspermsuk, E.; Chaisri, A.; Kiwwongngam, R.; Wuttimongkolkul, S.; Charoenkiatkul, S. Proximate Composition, Dietary Fibre, Beta-Glucan Content, and Inhibition of Key Enzymes Linked to Diabetes and Obesity in Cultivated and Wild Mushrooms. J. Food Compos. Anal. 2022, 105, 104226.
- Das, P.; Sikdar, S.R.; Samanta, A. Nutritional Analysis and Molecular Characterization of Hybrid Mushrooms Developed through Intergeneric Protoplast Fusion between Pleurotus Sajor-Caju and Calocybe indica with the Purpose to Achieve Improved Strains. World J. Microbiol. Biotechnol. 2021, 37, 69.
- Nayak, P.C.; Raju, C.V.; Lakshmisha, I.P.; Singh, R.R.; Sofi, F.R. Influence of Button Mushroom (Agaricus bisporus) on Quality and Refrigerated Storage Stability of Patties Prepared from Sutchi Catfish (Pangasius hypophthalmus). J. Food Sci. Technol. 2014.
- Jo, K.; Lee, J.; Jung, S. Quality Characteristics of Low-Salt Chicken Sausage Supplemented with a Winter Mushroom Powder. Korean J. Food Sci. Anim. Resour. 2018, 38, 768–779.
- Mau, J.-L.; Lin, H.-C.; Ma, J.-T.; Song, S.-F. Non-Volatile Taste Components of Several Speciality Mushrooms. Food Chem. 2001, 73, 461–466.
- Xu, Z.; Fu, L.; Feng, S.; Yuan, M.; Huang, Y.; Liao, J.; Zhou, L.; Yang, H.; Ding, C. Chemical Composition, Antioxidant and Antihyperglycemic Activities of the Wild Lactarius deliciosus from China. Molecules 2019, 24, 1357.
- Teklit, G.A. Chemical Composition and Nutritional Value of the Most Widely Used Mushrooms Cultivated in Mekelle Tigray Ethiopia. J. Nutr. Food Sci. 2015, 5, 1.
- Michael, H.W.; Bultosa, G.; Pant, L.M. Nutritional Contents of Three Edible Oyster Mushrooms Grown on Two Substrates at Haramaya, Ethiopia, and Sensory Properties of Boiled Mushroom and Mushroom Sauce: Nutrient of Edible Oyster Mushrooms. Int. J. Food Sci. Technol. 2011, 46, 732–738.
- Crisan, E.V.; Sands, A. Nutritional Value. In Biology and Cultivation of Edible Mushrooms; Chang, S.T., Hayer, W.A., Eds.; Academic Press: New York, NY, USA, 1978; pp. 138–168.
- Ghosh, K. A Review: Edible Mushrooms as Source of Dietary Fiber and Its Health Effects. J. Phys. Sci. 2016, 21, 129–137.
- Acar, İ.; Blando, F.; Gul, B.; Greco, A.; Mukemre, M.; Uzun, Y.; Dalar, A. The Phenolic Profile and Biological Activities of the Wild-Edible Mushrooms Helvella leucopus and Morchella pulchella. Food Meas. 2021, 15, 555–566.
- Alkin, M.; Söğüt, E.; Seydim, A.C. Determination of Bioactive Properties of Different Edible Mushrooms from Turkey. Food Meas. 2021, 15, 3608–3617.
- Siwulski, M.; Budka, A.; Rzymski, P.; Gąsecka, M.; Kalač, P.; Budzyńska, S.; Magdziak, Z.; Niedzielski, P.; Mleczek, P.; Mleczek, M. Worldwide Basket Survey of Multielemental Composition of White Button Mushroom Agaricus Bisporus. Chemosphere 2020, 239, 124718.
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, Medicinal, and Cosmetic Value of Bioactive Compounds in Button Mushroom (Agaricus bisporus): A Review. Appl. Sci. 2021, 11, 5943.
- Koutrotsios, G.; Tagkouli, D.; Bekiaris, G.; Kaliora, A.; Tsiaka, T.; Tsiantas, K.; Chatzipavlidis, I.; Zoumpoulakis, P.; Kalogeropoulos, N.; Zervakis, G.I. Enhancing the Nutritional and Functional Properties of Pleurotus citrinopileatus Mushrooms through the Exploitation of Winery and Olive Mill Wastes. Food Chem. 2022, 370, 131022.
- Saini, R.K.; Rauf, A.; Khalil, A.A.; Ko, E.-Y.; Keum, Y.-S.; Anwar, S.; Alamri, A.; Rengasamy, K.R.R. Edible Mushrooms Show Significant Differences in Sterols and Fatty Acid Compositions. S. Afr. J. Bot. 2021, 141, 344–356.
- Koç, E.; Karayiğit, B. Assessment of Biofortification Approaches Used to Improve Micronutrient-Dense Plants that Are a Sustainable Solution to Combat Hidden Hunger. J. Soil Sci. Plant Nutr. 2021, 4, 1–26.
- Kora, A.J. Nutritional and Antioxidant Significance of Selenium-Enriched Mushrooms. Bull. Natl. Res. Cent. 2020, 44, 34.
- Siwulski, M.; Budzyńska, S.; Rzymski, P.; Gąsecka, M.; Niedzielski, P.; Kalač, P.; Mleczek, M. The Effects of Germanium and Selenium on Growth, Metalloid Accumulation and Ergosterol Content in Mushrooms: Experimental Study in Pleurotus ostreatus and Ganoderma lucidum. Eur. Food Res. Technol. 2019, 245, 1799–1810.
- Hu, T.; Liang, Y.; Zhao, G.; Wu, W.; Li, H.; Guo, Y. Selenium Biofortification and Antioxidant Activity in Cordyceps militaris Supplied with Selenate, Selenite, or Selenomethionine. Biol. Trace Elem. Res. 2019, 187, 553–561.
- Hu, T.; Hui, G.; Li, H.; Guo, Y. Selenium Biofortification in Hericium erinaceus (Lion’s Mane Mushroom) and Its in Vitro Bioaccessibility. Food Chem. 2020, 331, 127287.
- Xu, M.; Zhu, S.; Wang, L.; Wei, Z.; Zhao, L.; Shi, G.; Ding, Z. Influence of Selenium Biofortification on the Growth and Bioactive Metabolites of Ganoderma lucidum. Foods 2021, 10, 1860.
- El-Ramady, H.; Abdalla, N.; Elbasiouny, H.; Elbehiry, F.; Elsakhawy, T.; Omara, A.E.-D.; Amer, M.; Bayoumi, Y.; Shalaby, T.A.; Eid, Y.; et al. Nano-Biofortification of Different Crops to Immune against COVID-19: A Review. Ecotoxicol. Environ. Saf. 2021, 222, 112500.
- He, L.; Zhao, J.; Wang, L.; Liu, Q.; Fan, Y.; Li, B.; Yu, Y.-L.; Chen, C.; Li, Y.-F. Using Nano-Selenium to Combat Coronavirus Disease 2019 (COVID-19)? Nano Today 2021, 36, 101037.
This entry is offline, you can click here to edit this entry!
