Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Crohn’s disease (CD) is a chronic progressive inflammatory bowel disease leading to bowel damage and disability. The diagnosing recurrence in postoperative Crohn's disease is discussed.
- surgery
- endoscopy
- biomarkers
1. Introduction
Crohn’s disease (CD) is a chronic progressive inflammatory bowel disease leading to bowel damage and disability [1]. The expanding treatment armamentarium and wider use of biologics has paralleled a decline in the rates of surgical resection [2][3]. Nonetheless, surgery remains an important treatment modality in patients with obstructive symptoms, penetrating complications, or medically refractory disease. Up to 25% of patients with CD undergo surgery within five years of diagnosis [4], and up to a quarter of these patients will undergo a second resection within five years of the first [5]. Managing post-operative CD is challenging and complex as many of its aspects are incompletely supported by evidence.
Society guidelines suggest stratification on clinical risk factors to guide prophylactic treatment after resection, followed by endoscopy at 6 months to inform potential treatment escalation [6][7][8]. Notably, risk factors for recurrence have never been prospectively validated and guidelines differ in the definition of a patient at high risk for recurrence, with the British Society of Gastroenterology requiring the presence of at least two risk factors [8], whilst the American Gastroenterological Association and the European Crohn’s and Colitis Organization mandate the presence of a single risk factor [6][7]. Endoscopy-based management is supported by the findings of the Post-Operative Crohn’s Endoscopic Recurrence (POCER) study, a randomized trial demonstrating the superiority of early colonoscopy compared to standard care [9]. In spite of the crucial role of endoscopy in the management of postoperative CD, no endoscopic index has been fully validated for this purpose, including the most widely used Rutgeerts score [10]. Further uncertainty surrounds the choice of therapeutic agent to prevent postoperative recurrence. Metronidazole and thiopurines continue to be used despite marginal efficacy over placebo and the potential for serious adverse events [11]. Although treatment with infliximab led to a statistically significant 28.9% reduction in the risk of endoscopic recurrence, the PREVENT trial did not meet its primary endpoint composed of clinical and endoscopic recurrence, as only 18.1% (20/110) of patients with endoscopic recurrence as defined by the Rutgeerts Score ≥ i2 also had recurrence based on the CD Activity Index (CDAI) (defined by a total CDAI score >200 and a ≥70-point increase from baseline) [12]. Consequently, none of the drugs used for the treatment of CD have received regulatory approval for prevention of postoperative recurrence.
2. Diagnosing Recurrence in Postoperative Crohn’s Disease
2.1. Endoscopy
Endoscopic assessment is the gold standard for diagnosing postoperative recurrence and is the cornerstone of decision-making in the postoperative period. A colonoscopy-based monitoring strategy was evaluated in the randomized POCER trial and was shown to be superior to conventional management in reducing the rate of recurrence at 18 months [9]. There is some disagreement as to what constitutes endoscopic recurrence, namely whether lesions confined to the anastomosis carry the same prognostic significance as lesions in the neoterminal ileum. In addition to the Rutgeerts score, two new endoscopic indices have recently been developed. A comparison of available endoscopic indices is presented in Figure 1.
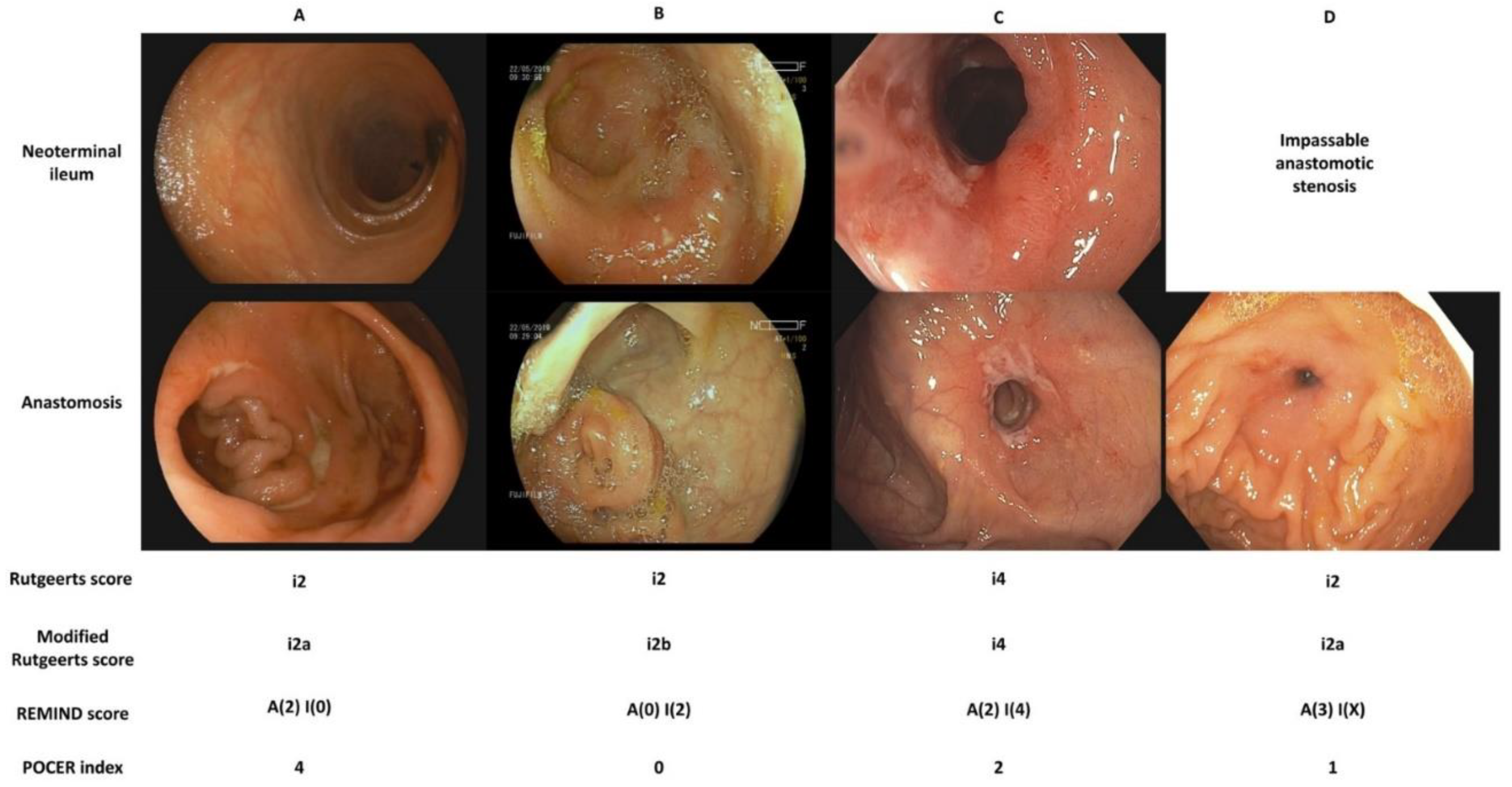
Figure 1. Comparison of endoscopic indices for the assessment of postoperative Crohn’s disease. (A). The neoterminal ileum is free of ulceration, two ulcers, one of them deeper than 2 mm, are present at the anastomosis and cover more than 50% of the circumference. (B). There are more than five aphthous ulcers with normal intervening mucosa in the neoterminal ileum. The anastomosis is free of ulceration. (C). The neoterminal ileum is diffusely inflamed with large ulcers. The anastomosis is superficially ulcerated along more than 50% of its circumference. (D). The anastomosis is impassable due to stenosis. A superficial ulcer covers less than 25% of its circumference. Note that an anastomotic stenosis should be scored as i2 on the Rutgeerts score and i2a on its modified version. Only a stenosis in the neoterminal ileum should be scored as i4.
2.1.1. (Modified) Rutgeerts Score
Society guidelines advocate endoscopic evaluation in all patients at 6 months after surgery. Endoscopic disease activity in the neoterminal ileum and the ileocolonic anastomosis has traditionally been evaluated using the Rutgeerts score (Table 1), where i1–i4 is considered endoscopic recurrence and escalation of therapy is recommend for scores of i2 an higher [10]. Despite its widespread use in clinical practice and clinical trials, the score’s operating characteristics have not been fully studied [13], with its responsiveness remaining unknown. The inter-rater reliability was shown to be “substantial” upon evaluation by expert endoscopists, although defining aphthous ulcers in the neoterminal ileum was a source of disagreement, potentially due to difficulty of separating small ulcers from mucus or residual debris.
Table 1. Endoscopic indices used for the assessment of postoperative Crohn’s disease.
| Rutgeerts Score [10] | |
| i0 | No lesions |
| i1 | ≤5 aphthous lesions in the neoterminal ileum |
| i2 | >5 aphthous lesions with normal intervening mucosa or skip area of large lesions or lesions confined to the ileo-colonic anastomosis |
| i3 | Diffuse aphthous ileitis with diffusely inflamed mucosa |
| i4 | Large ulcers with diffuse mucosal inflammation or nodules or stenosis in the neo-terminal ileum |
| Modified Rutgeerts Score [14] | |
| i0 | No lesions |
| i1 | ≤5 aphthous lesions in the neoterminal ileum |
| i2a | Lesions confined to the ileo-colonic anastomosis (including anastomotic stenosis) |
| i2b | >5 aphthous ulcers or large lesions, with normal mucosa in-between, in the neo-terminal ileum (with or without anastomotic lesions) |
| i3 | Diffuse aphthous ileitis with diffusely inflamed mucosa |
| i4 | Large ulcers with diffuse mucosal inflammation or nodules or stenosis in the neo-terminal ileum |
| REMIND Score [15] | |
| Anastomotic lesions (<1 cm in length after the anastomosis | |
| A (0) | No lesions |
| A (1) | Ulcerations covering less than 50% of the anastomosis circumference |
| A (2) | Ulcerations covering more than 50% of the anastomosis circumference |
| A (3) | Anastomotic stenosis |
| Ileal lesions | |
| I (0) | No lesions |
| I (1) | ≤5 aphthous lesions in the neoterminal ileum |
| I (2) | >5 aphthous lesions with normal intervening mucosa or skip areas of larger lesions |
| I (3) | Diffuse aphthous ileitis with diffusely inflamed mucosa |
| I (4) | Diffuse inflammation with larger ulcers |
| POCER Index [16] | |
| 0 | No anastomotic ulcers |
| 1 | Superficial anastomotic ulcers (<2 mm in depth), <25% circumferential extent |
| 2 | Superficial anastomotic ulcers (<2 mm in depth), ≥25% circumferential extent |
| 3 | Deep anastomotic ulcer (≥1 ulcer with ≥2 mm depth), <25% circumferential extent |
| 4 | Deep anastomotic ulcer (≥1 ulcer with ≥2 mm depth), ≥25% circumferential extent |
The hypothesis than anastomotic lesions portend a better prognosis compared to lesions in the neoterminal ileum resulted in the modified Rutgeerts score [14], which separates i2 into isolated lesions confined to the ileocolonic anastomosis (i2a), while all other lesions qualifying for i2 on the original score are classified as i2b (>5 aphthous ulcers or large lesions, with normal mucosa in-between, in the neo-terminal ileum, regardless of concomitant anastomotic lesions) (Table 1). Nonetheless, recent research has demonstrated histological features of CD, rather than ischemia, in the majority of anastomotic ulcers [17].
Comparisons between the original Rutgeerts score and its modification have yielded conflicting results for clinical outcomes [15][18][19][20][21]. The discrepancies could perhaps be explained by retrospective design of most studies and endoscopic assessment based on still images. The only prospective study evaluating the association of the modified Rutgeerts score with subsequent clinical outcomes was a French cohort study of 225 patients (193 with long-term follow-up) with local endoscopic reading [15]. The study indicated an incremental prognostic benefit of the modified score as clinical recurrence-free survival was similar between i0 and i2a, but significantly shorter for i2b compared to i0. These findings thus support the reasoning that isolated anastomotic ulcers have a better prognosis than ulcers in the neoterminal ileum.
2.1.2. REMIND Score
The REMIND score was developed in a French multicentric prospective study mentioned above [15]. This score separates anastomotic lesions (sub-score A) from ileal lesions (sub-score I), with anastomotic lesions graded based on their circumferential extent and ileal lesions as defined by the original Rutgeerts score (Table 1). The main finding of the study was that long-term outcomes were dependent on ileal, rather than anastomotic, lesions. Only the most severe anastomotic lesion, anastomotic stenosis, was associated with subsequent occlusive complications, but not clinical recurrence. A notable finding of the study was the high clinical recurrence rate in patients with I(1) lesions that did not differ significantly from recurrence rates with more severe ileal lesions. In summary, results from the REMIND cohort suggest that a lower threshold for escalating treatment should be applied to ileal lesions with treatment escalation at i1, rather than i2, while the presence of anastomotic lesions is a minor factor in the decision process.
Although good inter-rater reliability (weighted kappa coefficient of 0.82) was demonstrated in the original study, the score requires further validation in independent cohorts [22], particularly regarding its impact on treatment decisions in comparison with the Rutgeerts score.
2.1.3. Post-Operative Crohn’s Endoscopic Recurrence Index
The POCER index was developed on the subset of patients from the active arm of the POCER trial who had endoscopic assessment at 6 and 18 months (n = 85) [16]. Five new scoring items evaluated at the anastomosis were selected a priori to be assessed for their association with subsequent endoscopic recurrence (defined as a Rutgeerts score of i2 or greater): (1) total number of ulcers at the anastomosis; (2) ulcer depth; (3) circumferential extent of ulcers; (4) size of the largest ulcer; (5) presence of stenosis. None of the items were associated with subsequent endoscopic recurrence in isolation, but the anastomotic ulcer depth and circumference were selected to develop the new index based on factor analysis (Table 1).
2.2. Fecal and Serum Biomarkers
Despite being the gold standard, colonoscopy is invasive, requires bowel preparation and is not without risk. In fact, patients rated colonoscopy as the least acceptable monitoring tool [23]. In contrast, stool sampling, and, to an even greater extent, serum sampling are well accepted by patients and hold promise to be able to stratify patients by the risk for recurrence and individualize referrals for colonoscopy.
2.2.1. Fecal Biomarkers
Fecal calprotectin is a calcium- and zinc-binding protein expressed by neutrophils that is widely used for the noninvasive monitoring of CD [24]. Given that histologic changes preceding subsequent endoscopic recurrence are known to develop within days of surgery [25], fecal calprotectin could not only serve as a diagnostic biomarker (Does this patient have endoscopic recurrence?), but also a predictive biomarker (Will this patient develop endoscopic recurrence?).
Its performance in postoperative CD was evaluated by two meta-analyses [26][27]. Both meta-analyses defined a Rutgeerts score of ≥i2 as endoscopic recurrence. At a cutoff of 100 mcg/g, the sensitivity for endoscopic recurrence was 81% and the specificity 57%, at 150 mcg/g, the sensitivity was 70% and specificity 69% [27]. A positive association between calprotectin concentrations and the severity of endoscopic recurrence has been demonstrated [28]. By extension, in a study evaluating the performance of fecal calprotectin against both versions of the Rutgeerts score showed superior test characteristics (cutoff 100 mcg/g; sensitivity: 74% vs. 48%; specificity: 91% vs. 33%) with the modified Rutgeerts, reflecting the fact that calprotectin concentrations were lower in patients with i2a than i2b [29].
2.2.2. Serum Biomarkers
The Endoscopic healing index (EHI) is a recently validated assay measuring 13 serum proteins to noninvasively identify patients with CD in endoscopic remission (Simple endoscopic score for CD [SES-CD] ≤ 2) [30]. At a cutoff value of 20 points (calculated by a proprietary algorithm), the performance of EHI was similar to that of fecal calprotectin in the training and validation cohorts.
The EHI was measured for stored serum samples from the POCER trial [31]. At 6 months, an EHI ≤ 20 had a negative predictive value of 75.7% for endoscopic recurrence. At this time point, both fecal calprotectin and EHI performed similarly [31][32]. At 18 months postoperatively, however, the EHI could not discriminate between remission and recurrence, unlike fecal calprotectin which maintained a negative predictive value of 89.7% for a cutoff of 100 mcg/g. The cause for this discrepancy at 18 months is unknown and may potentially be related to the fact that the EHI was developed using the SES-CD and not the Rutgeerts score: a single aphthous ulcer in the neoterminal ileum (i1) would score 3 points on the SES-CD, as would 6 aphthous ulcers (i2), provided that the percentage of ulcerated or affected surface was below 10% and 50%, respectively. The authors also explored the possibility of using both tests in tandem, which resulted in a modest improvement in test characteristics. It is thus unclear whether performing both tests simultaneously improves diagnostic performance to a meaningful extent.
2.3. Cross-Sectional Imaging
With CD being a transmural disease, there is a lingering concern that endoscopic evaluation limited to the mucosa is inadequate to account for the full spectrum of disease, overlooking changes in the intestinal wall that could affect subsequent management. Cross-sectional imaging has the potential to overcome this limitation; moreover, it is noninvasive and neither ultrasound nor magnetic resonance imaging expose patients to ionizing radiation, making it an attractive monitoring tool for postoperative CD.
2.3.1. Intestinal Ultrasound
DiCandio et al. [33] were the first to use intestinal ultrasound to diagnose postoperative recurrence of CD in 1986—four years before the publication of the Rutgeerts score. Different ultrasonographic techniques have been used: bowel sonography without the use of intravenous or oral contrast, small intestine contrast ultrasound with the use of oral contrast solution, and contrast-enhanced ultrasound using an intravenous contrast medium. Oral contrast solution serves to facilitate assessment by distending bowel loops, while intravenous contrast enables the assessment of vascularization and hyperemia in active CD.
2.3.2. Magnetic Resonance and Computed Tomography Enterography
The sensitivity of enterography to detect endoscopic recurrence has ranged from 92 to 96% and its specificity from 75 to 88% [34][35][36][37]. In line with the notion that mucosal visualization during colonoscopy provides an incomplete appraisal of disease burden, a recent study explored the concordance between radiographic and endoscopic findings [38]. In this retrospective cohort study, the images of 216 postoperative patients with enterography and colonoscopy performed within 90 days of each other were reviewed. Endoscopic recurrence was defined as ≥i2b. The majority of patients, 54.2% (117/216), had concordant findings between radiology and endoscopy, 41.7% (90/216) had radiological, but not endoscopic signs of active disease, and 4.2% (9/216) had endoscopic, but not radiological signs of active disease. Notably, patients with radiological, but not endoscopic, disease activity had a shorter time to endoscopic recurrence and greater risk of surgical recurrence.
These findings seem concerning, as they suggest that endoscopic assessment systematically underestimates the risk for recurrence, thereby questioning the validity of a monitoring approach based on endoscopy. The majority of discrepant results are readily explained by the cut-off for endoscopic recurrence of i2b in the study: of the 90 patients with radiologic, but not endoscopic, signs of recurrence, 62.2% (56/90) had endoscopically active disease which did not fulfil criteria for recurrence in the study (43 patients with i2a; 13 patients with i1). Proximal small bowel disease was the reason for discordant findings in only three patients. In a sensitivity analysis, where the threshold for endoscopic recurrence was set at i2a, there was no longer a significant difference of subsequent endoscopic recurrence between patients with no radiologic or endoscopic signs of recurrence (46.2%) and patients with radiologic, but not endoscopic, signs of recurrence (55.6%). In summary, the results of this study highlight the gradient of risk for recurrence from i1, across i2a to i2b, rather than an important intrinsic difference between radiologic and endoscopic monitoring strategies that would lead to consequences for patient management.
2.4. Novel and Emerging Biomarkers
Emerging biomarkers for the prediction and diagnosis of postoperative recurrence include single nucleotide polymorphisms (SNPs), transcriptomics, metabolomics and microbial markers. These could facilitate the decision to institute postoperative prophylactic therapy.
In a retrospective cohort of 372 patients with CD undergoing surgery, a polymorphism in transcription factor 4 (TCF4) conferred a significant risk of surgical recurrence (OR 4.10; 95% CI 2.37–7.11) [39]. In a study of 60 patients, RNA was extracted from the noninflamed ileal margin of resection specimens, the transcripts were later classified by random forest, a machine learning algorithm, to identify patients with i0 endoscopic scores [40]. In anti-TNF naïve patients, a clear transcriptional cluster separating patients with i0 scores from other patients was identified. In anti-TNF exposed patients, no association between transcriptional profiles and endoscopic scores were found. The investigators developed an ad hoc score to define an indolent disease course after surgery, which was associated with distinct transcriptional profiles even in anti-TNF experienced patients. In a small prospective study of 38 patients, elevated urinary levoglucosan concentrations were associated with endoscopic recurrence [41]. Levoglucosan concentrations were a diagnostic (i.e., recurrence had already occurred), rather than predictive, biomarker and it remains to be determined whether this biomarker offers an advantage compared to fecal calprotectin.
This entry is adapted from the peer-reviewed paper 10.3390/jcm11226746
References
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755.
- Tsai, L.; Ma, C.; Dulai, P.S.; Prokop, L.J.; Eisenstein, S.; Ramamoorthy, S.L.; Feagan, B.G.; Jairath, V.; Sandborn, W.J.; Singh, S. Contemporary Risk of Surgery in Patients With Ulcerative Colitis and Crohn’s Disease: A Meta-Analysis of Population-Based Cohorts. Clin. Gastroenterol. Hepatol. 2020, 19, 2031–2204.
- Ma, C.; Moran, G.W.; Benchimol, E.I.; Targownik, L.E.; Heitman, S.J.; Hubbard, J.N.; Seow, C.H.; Novak, K.L.; Ghosh, S.; Panaccione, R.; et al. Surgical Rates for Crohn’s Disease are Decreasing: A Population-Based Time Trend Analysis and Validation Study. Am. J. Gastroenterol. 2017, 112, 1840–1848.
- Frolkis, A.D.; Dykeman, J.; Negron, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006.
- Frolkis, A.D.; Lipton, D.S.; Fiest, K.M.; Negron, M.E.; Dykeman, J.; deBruyn, J.; Jette, N.; Frolkis, T.; Rezaie, A.; Seow, C.H.; et al. Cumulative incidence of second intestinal resection in Crohn’s disease: A systematic review and meta-analysis of population-based studies. Am. J. Gastroenterol. 2014, 109, 1739–1748.
- Gionchetti, P.; Dignass, A.; Danese, S.; Magro Dias, F.J.; Rogler, G.; Lakatos, P.L.; Adamina, M.; Ardizzone, S.; Buskens, C.J.; Sebastian, S.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J. Crohn’s Colitis 2017, 11, 135–149.
- Nguyen, G.C.; Loftus, E.V., Jr.; Hirano, I.; Falck-Ytter, Y.; Singh, S.; Sultan, S. American Gastroenterological Association Institute Guideline on the Management of Crohn’s Disease After Surgical Resection. Gastroenterology 2017, 152, 271–275.
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106.
- De Cruz, P.; Kamm, M.A.; Hamilton, A.L.; Ritchie, K.J.; Krejany, E.O.; Gorelik, A.; Liew, D.; Prideaux, L.; Lawrance, I.C.; Andrews, J.M.; et al. Crohn’s disease management after intestinal resection: A randomised trial. Lancet 2015, 385, 1406–1417.
- Rutgeerts, P.; Geboes, K.; Vantrappen, G.; Beyls, J.; Kerremans, R.; Hiele, M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990, 99, 956–963.
- Burr, N.E.; Hall, B.; Hamlin, P.J.; Selinger, C.P.; Ford, A.C.; O’Connor, A. Systematic Review and Network Meta-Analysis of Medical Therapies to Prevent Recurrence of Post-Operative Crohn’s Disease. J. Crohn’s Colitis 2019, 13, 693–701.
- Regueiro, M.; Feagan, B.G.; Zou, B.; Johanns, J.; Blank, M.A.; Chevrier, M.; Plevy, S.; Popp, J.; Cornillie, F.J.; Lukas, M.; et al. Infliximab Reduces Endoscopic, but Not Clinical, Recurrence of Crohn’s Disease After Ileocolonic Resection. Gastroenterology 2016, 150, 1568–1578.
- Ma, C.; Gecse, K.B.; Duijvestein, M.; Sandborn, W.J.; Zou, G.; Shackelton, L.M.; Stitt, L.W.; Parker, C.E.; Bossuyt, P.; Lowenberg, M.; et al. Reliability of Endoscopic Evaluation of Postoperative Recurrent Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 2139–2141.
- Domenech, E.; Manosa, M.; Bernal, I.; Garcia-Planella, E.; Cabre, E.; Pinol, M.; Lorenzo-Zuniga, V.; Boix, J.; Gassull, M.A. Impact of azathioprine on the prevention of postoperative Crohn’s disease recurrence: Results of a prospective, observational, long-term follow-up study. Inflamm. Bowel Dis. 2008, 14, 508–513.
- Hirten, R.P.; Mashiana, S.; Cohen, B.L.; Sands, B.E.; Colombel, J.F.; Harpaz, N. Ileocolic anastomotic inflammation after resection for Crohn’s disease indicates disease recurrence: A histopathologic study. Scand. J. Gastroenterol. 2020, 55, 795–799.
- Ollech, J.E.; Aharoni-Golan, M.; Weisshof, R.; Normatov, I.; Sapp, A.R.; Kalakonda, A.; Israel, A.; Glick, L.R.; Karrison, T.; Dalal, S.R.; et al. Differential risk of disease progression between isolated anastomotic ulcers and mild ileal recurrence after ileocolonic resection in patients with Crohn’s disease. Gastrointest. Endosc. 2019, 90, 269–275.
- Riviere, P.; Vermeire, S.; Irles-Depe, M.; Van Assche, G.; Rutgeerts, P.; de Buck van Overstraeten, A.; Denost, Q.; Wolthuis, A.; D’Hoore, A.; Laharie, D.; et al. No Change in Determining Crohn’s Disease Recurrence or Need for Endoscopic or Surgical Intervention With Modification of the Rutgeerts’ Scoring System. Clin. Gastroenterol. Hepatol. 2019, 17, 1643–1645.
- Hammoudi, N.; Auzolle, C.; Tran Minh, M.L.; Boschetti, G.; Bezault, M.; Buisson, A.; Pariente, B.; Treton, X.; Seksik, P.; Fumery, M.; et al. Postoperative Endoscopic Recurrence on the Neoterminal Ileum But Not on the Anastomosis Is Mainly Driving Long-Term Outcomes in Crohn’s Disease. Am. J. Gastroenterol. 2020, 115, 1084–1093.
- Hirten, R.P.; Ungaro, R.C.; Castaneda, D.; Lopatin, S.; Sands, B.E.; Colombel, J.F.; Cohen, B.L. Anastomotic Ulcers After Ileocolic Resection for Crohn’s Disease Are Common and Predict Recurrence. Inflamm. Bowel Dis. 2020, 26, 1050–1058.
- Bachour, S.P.; Shah, R.S.; Lyu, R.; Rieder, F.; Qazi, T.; Lashner, B.; Achkar, J.P.; Philpott, J.; Barnes, E.L.; Axelrad, J.; et al. Mild neoterminal ileal post-operative recurrence of Crohn’s disease conveys higher risk for severe endoscopic disease progression than isolated anastomotic lesions. Aliment. Pharmacol. Ther. 2022, 55, 1139–1150.
- Riviere, P.; Pekow, J.; Hammoudi, N.; Wils, P.; De Cruz, P.; Wang, C.P.; Manosa, M.; Ollech, J.; Allez, M.; Nachury, M.; et al. Comparison of the risk of Crohn’s disease postoperative recurrence between modified Rutgeerts score i2a and i2b categories: An individual patient data meta-analysis. J. Crohn’s Colitis 2022, jjac137.
- De Cruz, P.; Hamilton, A.L.; Burrell, K.J.; Gorelik, A.; Liew, D.; Kamm, M.A. Endoscopic Prediction of Crohn’s Disease Postoperative Recurrence. Inflamm. Bowel Dis. 2021, 28, 680–688.
- Buisson, A.; Gonzalez, F.; Poullenot, F.; Nancey, S.; Sollellis, E.; Fumery, M.; Pariente, B.; Flamant, M.; Trang-Poisson, C.; Bonnaud, G.; et al. Comparative Acceptability and Perceived Clinical Utility of Monitoring Tools: A Nationwide Survey of Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1425–1433.
- D’Amico, F.; Rubin, D.T.; Kotze, P.G.; Magro, F.; Siegmund, B.; Kobayashi, T.; Olivera, P.A.; Bossuyt, P.; Pouillon, L.; Louis, E.; et al. International consensus on methodological issues in standardization of fecal calprotectin measurement in inflammatory bowel diseases. United Eur. Gastroenterol. J. 2021, 9, 451–460.
- D’Haens, G.R.; Geboes, K.; Peeters, M.; Baert, F.; Penninckx, F.; Rutgeerts, P. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998, 114, 262–267.
- Qiu, Y.; Mao, R.; Chen, B.L.; He, Y.; Zeng, Z.R.; Xue, L.; Song, X.M.; Li, Z.P.; Chen, M.H. Fecal calprotectin for evaluating postoperative recurrence of Crohn’s disease: A meta-analysis of prospective studies. Inflamm. Bowel Dis. 2015, 21, 315–322.
- Tham, Y.S.; Yung, D.E.; Fay, S.; Yamamoto, T.; Ben-Horin, S.; Eliakim, R.; Koulaouzidis, A.; Kopylov, U. Fecal calprotectin for detection of postoperative endoscopic recurrence in Crohn’s disease: Systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2018, 11, 175628481878557.
- Boschetti, G.; Laidet, M.; Moussata, D.; Stefanescu, C.; Roblin, X.; Phelip, G.; Cotte, E.; Passot, G.; Francois, Y.; Drai, J.; et al. Levels of Fecal Calprotectin Are Associated With the Severity of Postoperative Endoscopic Recurrence in Asymptomatic Patients With Crohn’s Disease. Am. J. Gastroenterol. 2015, 110, 865–872.
- Lopes, S.; Andrade, P.; Afonso, J.; Rodrigues-Pinto, E.; Dias, C.C.; Macedo, G.; Magro, F. Correlation Between Calprotectin and Modified Rutgeerts Score. Inflamm. Bowel Dis. 2016, 22, 2173–2181.
- D’Haens, G.; Kelly, O.; Battat, R.; Silverberg, M.S.; Laharie, D.; Louis, E.; Savarino, E.; Bodini, G.; Yarur, A.; Boland, B.S.; et al. Development and Validation of a Test to Monitor Endoscopic Activity in Patients with Crohn’s Disease Based on Serum Levels of Proteins. Gastroenterology 2020, 158, 515–526.e10.
- Hamilton, A.L.; De Cruz, P.; Wright, E.K.; Dervieux, T.; Jain, A.; Kamm, M.A. Non-Invasive Serological Monitoring for Crohn’s Disease Post-Operative Recurrence. J. Crohn’s Colitis 2022, jjac076.
- Wright, E.K.; Kamm, M.A.; De Cruz, P.; Hamilton, A.L.; Ritchie, K.J.; Krejany, E.O.; Leach, S.; Gorelik, A.; Liew, D.; Prideaux, L.; et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology 2015, 148, 938–947.e1.
- DiCandio, G.; Mosca, F.; Campatelli, A.; Bianchini, M.; D’Elia, F.; Dellagiovampaola, C. Sonographic detection of postsurgical recurrence of Crohn disease. AJR Am. J. Roentgenol. 1986, 146, 523–526.
- Soyer, P.; Boudiaf, M.; Sirol, M.; Dray, X.; Aout, M.; Duchat, F.; Vahedi, K.; Fargeaudou, Y.; Martin-Grivaud, S.; Hamzi, L.; et al. Suspected anastomotic recurrence of Crohn disease after ileocolic resection: Evaluation with CT enteroclysis. Radiology 2010, 254, 755–764.
- Paparo, F.; Revelli, M.; Puppo, C.; Bacigalupo, L.; Garello, I.; Garlaschi, A.; Biscaldi, E.; Rollandi, L.; Binda, G.A.; Rollandi, G.A. Crohn’s disease recurrence in patients with ileocolic anastomosis: Value of computed tomography enterography with water enema. Eur. J. Radiol. 2013, 82, e434–e440.
- Mao, R.; Gao, X.; Zhu, Z.H.; Feng, S.T.; Chen, B.L.; He, Y.; Cui, Y.; Li, Z.P.; Hu, P.J.; Chen, M.H. CT enterography in evaluating postoperative recurrence of Crohn’s disease after ileocolic resection: Complementary role to endoscopy. Inflamm. Bowel Dis. 2013, 19, 977–982.
- Choi, I.Y.; Park, S.H.; Park, S.H.; Yu, C.S.; Yoon, Y.S.; Lee, J.L.; Ye, B.D.; Kim, A.Y.; Yang, S.K. CT Enterography for Surveillance of Anastomotic Recurrence within 12 Months of Bowel Resection in Patients with Crohn’s Disease: An Observational Study Using an 8-Year Registry. Korean J. Radiol. 2017, 18, 906–914.
- Bachour, S.P.; Shah, R.S.; Lyu, R.; Nakamura, T.; Shen, M.; Li, T.; Dane, B.; Barnes, E.L.; Rieder, F.; Cohen, B.; et al. Test Characteristics of Cross-sectional Imaging and Concordance with Endoscopy in Postoperative Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2021, 20, 2327–2336.e4.
- Wang, M.H.; Friton, J.J.; Raffals, L.E.; Leighton, J.A.; Pasha, S.F.; Picco, M.F.; Monroe, K.; Nix, B.D.; Newberry, R.D.; Faubion, W.A. Novel Genetic Variant Predicts Surgical Recurrence Risk in Crohn’s Disease Patients. Inflamm. Bowel Dis. 2021, 27, 1968–1974.
- Cushing, K.C.; McLean, R.; McDonald, K.G.; Gustafsson, J.K.; Knoop, K.A.; Kulkarni, D.H.; Sartor, R.B.; Newberry, R.D. Predicting Risk of Postoperative Disease Recurrence in Crohn’s Disease: Patients With Indolent Crohn’s Disease Have Distinct Whole Transcriptome Profiles at the Time of First Surgery. Inflamm. Bowel Dis. 2019, 25, 180–193.
- Keshteli, A.H.; Tso, R.; Dieleman, L.A.; Park, H.; Kroeker, K.I.; Jovel, J.; Gillevet, P.M.; Sikaroodi, M.; Mandal, R.; Fedorak, R.N.; et al. A Distinctive Urinary Metabolomic Fingerprint Is Linked With Endoscopic Postoperative Disease Recurrence in Crohn’s Disease Patients. Inflamm. Bowel Dis. 2018, 24, 861–870.
This entry is offline, you can click here to edit this entry!
