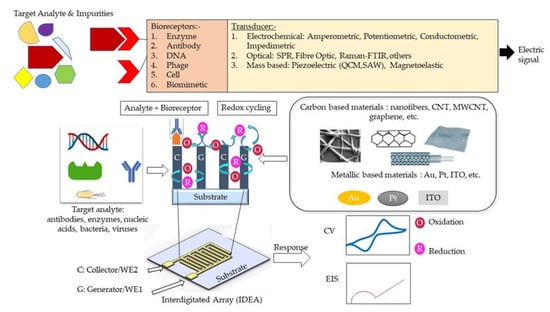
Micro and nano interdigitated electrode array (µ/n-IDEA) configurations are prominent working electrodes in the fabrication of electrochemical sensors/biosensors, as their design benefits sensor achievement. This paper reviews µ/n-IDEA as working electrodes in four-electrode electrochemical sensors in terms of two-dimensional (2D) planar IDEA and three-dimensional (3D) IDEA configurations using carbon or metal as the starting materials. In this regard, the enhancement of IDEAs-based biosensors focuses on controlling the width and gap measurements between the adjacent fingers and increases the IDEA’s height. Several distinctive methods used to expand the surface area of 3D IDEAs, such as a unique 3D IDEA design, integration of mesh, microchannel, vertically aligned carbon nanotubes (VACNT), and nanoparticles, are demonstrated and discussed. More notably, the conventional four-electrode system, consisting of reference and counter electrodes will be compared to the highly novel two-electrode system that adopts IDEA’s shape. Compared to the 2D planar IDEA, the expansion of the surface area in 3D IDEAs demonstrated significant changes in the performance of electrochemical sensors. Furthermore, the challenges faced by current IDEAs-based electrochemical biosensors and their potential solutions for future directions are presented herein.
[1]1. Introduction
A biosensor typically consists of three main elements, namely a bioreceptor, transducer, and signal processing system [
1]. A bioreceptor, also known as biological recognition element, involves an immobilized biocomponent, which is capable of detecting a specific target analyte [
2]. Nucleic acid, enzymes, antibodies, cells, etc., are types of biocomponents. A transducer is a converter that converts a biochemical signal into an electrical signal [
3]. The reaction between a bioreceptor and target analytes generates distinct chemical reactions, such as electron flow, release of heat, and changes in pH or mass, subsequently creating new chemicals. The detection of an electrical signal by the transducer is amplified and sent to microelectronics and data processors for signal measurement in terms of a print out, an optical change, or as digital display. Biosensors can be categorized into bioreceptors and transducers. Bioreceptors consist of biomimetics, enzymes, phages, DNA, cells, and antibodies. The transducer can be further divided into three categories, which are: (i) those based on electrochemical transducers, such as electrical impedance spectroscopy (EIS), potentiometric, amperometric, and conductometric; (ii) mass-based, such as piezoelectric and magnetoelastic; and (iii) optical-based biosensors, such as chemiluminescence, fluorescence, surface plasmon resonance (SPR), fibre optic, and others. The classification of biosensors has been described in several pieces of literature and is illustrated in
Figure 1 [
4,
5,
6].
Figure 1. Schematic design of IDEA design-based electrochemical detection (amperometric and impedimetric) methods.
The electrochemical biosensing techniques can be divided into amperometric, impedimetric, conductometric, and potentiometric sensing [
5]. In an amperometric sensor, one measures the current response at a fixed potential to detect the concentration of an analyte [
7]. In a potentiometric sensor, potential changes at a working/sensing electrode are measured with respect to a reference electrode and under the conditions of constant current (i.e., typically zero). Conductometric sensors measure the electrolytic conductivity to monitor the progress of a reaction. An impedimetric sensor works by measuring an impedance change while applying a small sinusoidal voltage that is varied over a range of frequencies. Electrochemical sensors are among the most popular biosensors due to their simplicity, good-to-excellent limit of detection (LOD), high selectivity, and ease of fabrication, as well as the promising opportunity for miniaturization and low-cost fabrication [
6,
8,
9,
10]. Innovative electrodes in electrochemical cells can be mass-manufactured using a variety of materials and economical manufacturing processes [
11,
12,
13]. Moreover, integrated circuit technologies make it possible to integrate electrodes with electronics for further biosensor miniaturization [
14,
15].
A basic electrochemical three-electrode cell consists of a working electrode, a counter electrode, and a reference electrode. The working electrode is the actual transduction element for the electrochemical reaction at the electrode/analyte solution interface. A current at the working electrode is offset by an equal but opposite current at the counter electrode, and hence there is no current flow between the working and high-input impedance reference electrode, allowing people to accurately track changes in the working electrode potential. The counter electrode must be of large surface area (since the current through the cell must be controlled by the reaction at the working electrode) with a stable and good conductor [
16]. Carbon [
17], platinum (Pt) [
18], gold (Au) [
19], and other materials [
7,
20,
21] are the common materials used to fabricate the counter electrode. Reference electrodes are designed so that an equilibrium is set up with a known potential between a metal wire and the surrounding solution. A silver/silver chloride (Ag/AgCl) system is widely used as a reference electrode [
22,
23].
Despite the booming interests in IDEAs that started more than 30 years ago, current studies manipulate materials from different sources to suit the electrode usage of IDEA. Interdigitated electrode array (IDEA) from carbon and metal sources, in contrast, emerges as a favorable electrode biosensor for various health-monitoring and biomedical applications. Indeed, numerous pieces of research highlighting the development of an IDEAs-based biosensor with various detection methods have been extensively reported [
24,
25,
26]. Enhanced signal amplification allowing detection of low-concentration bioanalytes displayed by an IDEAs-based biosensor proved to be advantageous for electrochemical biosensing [
27,
28,
29]. With an IDEA configuration, the limit of detection (LOD) of a biosensor can be significantly improved [
30]. In such a configuration, two comb-shaped working electrodes are arranged in an interdigitated manner, as presented in
Figure 1 [
31,
32]. IDEAs are widely used as impedimetric transducers in electrochemical impedance spectroscopy (EIS) [
33,
34,
35]. EIS employs a controlled alternating current (AC) with electrical stimulus between 5 and 10 mV to measure small variations in capacitance/resistance caused by analyte and electrode surface interactions. These capacitance and resistance changes are due to changes in faradaic (electron transfer/resistance changes) and non-faradaic (dielectric/capacitance changes) processes at the electrode surface [
36]. The signal strength of an IDEA-based biosensor can be controlled through the optimization of the active area, width, and spacing of the electrode fingers [
37,
38,
39,
40]. Hence, they can be used for real-time, label-free, and in situ detection of target analytes [
41]. However, the main drawback of these EIS-based sensors is their poor detection limit, compared to other electrochemical methods [
5]. Contrastingly, a combination of electrochemical sensing techniques, for example, amperometry and impedimetry could enhance biosensor performance [
42]. The details on different electrochemical detection methods are presented in
Table 1.
When IDEAs are used in an amperometric redox amplifying biosensors, the interdigitated combs/fingers are called generator and collector electrodes. Redox cycling [
43,
44] occurs when redox species generated at the generator electrode (in an oxidation reaction) are collected at the collector electrode (in a reduction reaction) [
45]. For this to occur, the generator and collector electrodes must be spaced close enough to overlap the diffusion layers of the redox species between the two electrodes [
30,
46]. A small gap between the finger electrodes allows for reversible redox species to undergo repeated oxidations/reductions (redox-cycling) before diffusing out to the bulk solution. The redox amplification factor is the ratio of the generator current in dual-mode operation (i.e., the generator and collector are at different enough potentials to allow for redox amplification) to the generator current in single mode (the generator and collector are at the same potential). The collection efficiency [
47,
48] is the ratio of the collector current to the generator current [
49,
50] or the ratio of the cathodic current to the anodic current at steady state [
51]. The smaller the gap between adjacent comb electrodes, the shorter the diffusion time for the redox species to diffuse across the gap, resulting in a higher current amplification factor [
46,
49,
52]. Due to redox amplification, IDEA-based electrochemical biosensors exhibit high signal-to-noise ratios, and thus better LODs, low ohmic drops, and rapid response time [
53]. Furthermore, the efficiency of redox cycling and redox amplification factors can be further improved by increasing the height of the two working electrodes in a three-dimensional form [
54]. The three-dimensional IDEAs (3D IDEAs) with their higher aspect ratio improve the contribution of linear diffusion between the electrode sidewalls and increase the IDEA’s electrode surface overall area [
49]. Another favorable microfabrication strategy, called the carbon microelectromechanical systems (C-MEMS) [
55], offers the fabrication of high aspect ratio carbon IDEAs and is rendered simple and inexpensive through a one-step photolithography step of a polymer carbon precursor and subsequent pyrolysis [
54,
56].
2. Features in IDEA
2.1. Electrical Double Layer (EDL)
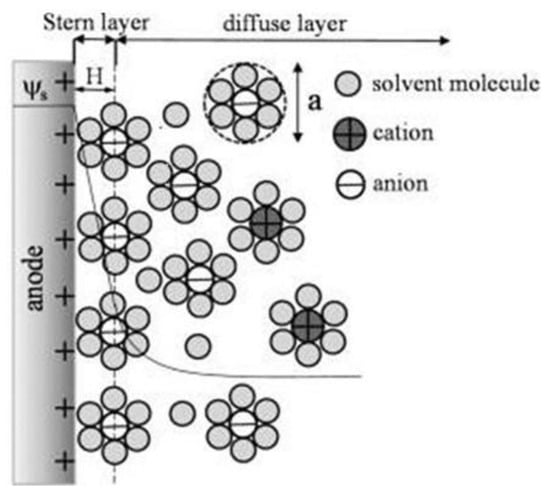
Figure 1 shows how the electrochemical IDEA-based sensor requires the interaction between target analyte/bioreceptor/electrolytic solutions and the sensor surface to produce an electrical signal. However, due to the various material sources for producing the electrode, such as carbon and metal, the interactions between the electrolytic solutions with the sensor create a phenomenon called electrical double layer (EDL) on the electrode surface [
64,
65,
66,
67,
68]. The EDL is formed when the electrons in the electrode surface (e.g., metal) interact with ions in the electrolyte solution. Referring to the Gouy–Chapman–Stern (GCS) EDL model, the liquid solution creates two layers consisting of a compact layer and a diffuse layer [
69]. The compact layer consists of the immobile solvent ions and molecules that adsorb into the solution/material interface, whereas the diffuse layer contains the mobile solution that carries solvated electroactive and inactive ions or the net charge within the liquid solution (
Figure 2). The scattering of charges in the diffuse layer is determined by the Debye length, thus, providing the surface potential or charge of the material [
70]. The EDL affected the electrochemical performance.
Yang et al. utilized computer simulation to study the effect of EDL in nanometer single electrode structure via the voltammetric performance. They reported that the extension of the diffuse layer into the diffusion layer in the EDL caused the increasing charge valence or the absence of the supporting electrolyte in the solution, which affected the current response of the nanometer electrode. It was reported that modifications to the thickness of the EDL compact layer and its relative permittivity significantly influenced the current response [
68,
69,
71]. Moreover, the ultramicroelectrodes, ranging from 25 µm to the submicrometer employed in published electrochemical experiments, were observed to cause nonlinear diffusion effects, resulting in enhanced mass transport, higher steady-state redox reaction rates, and faster response time, compared to larger electrodes [
50,
72,
73,
74].
Figure 2. Schematic of the electric double layer structure showing the arrangement of solvated anions and cations near the electrode/electrolyte interface in the Stern layer and the diffuse layer of Gouy–Chapman–Stern model. Reprinted with permission from Ref. [
75]. Copyright © 2022, American Chemical Society.
2.2. Crucial Parameters of the Sensor
Generally, the important parameters in performing biosensing are determined by sensitivity, selectivity, specificity, and limit of detection (LOD). In particular, the labelling method for target molecules is necessary for specificity, whereas the label-free method is more common for electrochemical detection using redox reaction [
76]. The sensitivity of the electrochemical measurement method depends on the relations among the electron transfer between the molecule and the electrode, creating significant amounts of electrical current without any label.
In IDEA, the width and gap between adjacent fingers are crucial in the fabrication of IDEA-based electrochemical sensors. A smaller width will result in smaller capacitance, and hence faster mass transport of the species. In the pursuit to enhance the sensitivity of IDEA-based biosensors with lower detection limits, the design of IDEA itself offers practical manipulation in terms of increasing the number of widths within the array or lengthening the width. As a result, simple modifications may lead to higher faradaic current to capacitive current ratios and higher signal-to-noise ratios, producing highly sensitive biosensors suitable for biosensing applications. Furthermore, reducing the gap between adjacent fingers leads to a higher diffusional flux of redox species and enhances the rapid response at the collector, as well as sensor sensitivity [
77].
Another factor to improve sensitivity of the biosensors for amperometric detection is redox cycling. Redox cycling between the narrow gap of the generator and collector of IDEA increase current response and signal-to-noise ratio [
69,
78]. A notable example was shown through work by Huang et al. [
79] in which smaller gaps between electrodes, such as a 300 nm gap, were required for the dopamine detection of 2.89 nA/µM to achieve high feedback between the generator and collector electrode. The smaller gap size between adjacent fingers may enhance the current amplification due to the shorter diffusion time between the two electrodes’ fingers [
46,
52,
80].
Redox amplification also improves the selectivity of the biosensor, as it can selectively amplify reversible redox species of interest over irreversible species [
30,
49]. The specificity of the biosensor can be determined by the specificity of the biologically active materials and the target analytes. In this case, a probe incorporated with targeted biological components and a transducer convert the biochemical signals into electrochemical, acoustic, etc., [
81]. In comparison, the optical method requires fluorescence detection or chemiluminescence with labels for biosensing detection. For example, green fluorescent protein (GFP) is a powerful tool to genetically encode a protein of interest (POI) for protein-based detection.
This entry is adapted from the peer-reviewed paper 10.3390/nano12234171