Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Exerkines are a group of promising molecules that may underlie the beneficial effects of physical exercise in diseases. The idea of exerkines is to understand the effects of physical exercise on diseases better. Exerkines have a high potential for the treatment of diseases and, considering that, there is still no study of the importance of exerkines on the most dangerous disease in the world in recent years, COVID-19.
- coronavirus disease 2019 (COVID-19)
- females
- exercise
- physical activity
- physical exercise
- exerkines
- exerkine
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the acute respiratory syndrome with coronavirus that primarily affects the lungs [1]. After three years of this disease, we understand who is more exposed to the symptoms of COVID-19 and who suffers from moderate and high severity of this disease [2]. The review of previous studies showed that most people infected with COVID-19 were obese or overweight [3][4], and disease severity and mortality were higher in obese subjects than in overweight subjects [5]. Moreover, during the illness, COVID-19 patients face a decrease in muscle strength (muscle atrophy), a decrease in muscle endurance, and other physiological factors, which can affect training programs in the long term after discharge from the hospital [6][7]. It has been determined that, in the long term, COVID-19 can lead to negative physiological changes and decrease the quality of life, reducing hope and motivation for physical activity, which worsens the course of the disease [8].
Gender is an essential determinant of mortality risk and immunological responses to COVID-19 [9][10][11]. Women face a higher risk of becoming infected during a pandemic because of their societal position, as the United Nations (UN) and the World Health Organization (WHO) reported [12][13][14]. Women are over-represented in health care professions [11][15][16][17]. On the other hand, obese and overweight women are more exposed to the complications of COVID-19 than normal women [18]. Being physically active or the lack thereof plays a vital role in obesity. Indubitably, obesity ensues from physical inactivity and vice versa [19][20][21]. Hormones can also affect sexual difference in viral infections such as COVID-19. The SARS-CoV-2 spike protein binds to the human angiotensin-converting enzyme 2 (ACE2) receptor. Studies have shown that estradiol, a primary female sex hormone, likely regulates ACE2 expression in airway epithelial cells, kidneys, heart, and adipose tissues [22][23]. In obese and overweight women, estradiol function [24] is disrupted and ACE2 expression increases [22][23].
Undeniable evidence has been supporting the vital role of physical exercise in improving immune system, body composition, and reducing the complications of COVID-19 [25][26]. Although the benefits of physical exercise in improving health and reducing the severity of COVID-19 are well known, the molecular mechanisms underlying these benefits associated with physical exercise are not yet defined and are being investigated [27][28]. Physical exercise generally includes aerobic, anaerobic, or resistance training, but physical activity includes occupational, sports, conditioning, household, or other activities [29][30][31]. Promoting physical activity seems to have a more significant effect on reducing the diseases severity [27][32][33][34]. In 2020, WHO reported that all adults should aim for 150 to 300 min of moderate-intensity physical activity per week or 75 to 150 min of vigorous-intensity physical activity per week, or an equivalent combination of moderate- and vigorous-intensity physical activity [35]. However, it is important to understand how physical activity can affect the acute or chronic immune response in obese women.
To make better recommendations for immunization in women, it is needed to understand the impact of physical exercise on all organs. Studying exerkines would be the key. The word exerkines has introduced in 2016 [36]. So far, there is no exact definition of exerkines, but the best explanation for seems to be: the release of myokines, cardiokines, hepatokines, and adipokines due to physical exercise [27]. That is, physical exercise can affect these “kines” and ultimately exerts its effects through endocrine, paracrine, and/or autocrine pathways (Figure 1) [35].
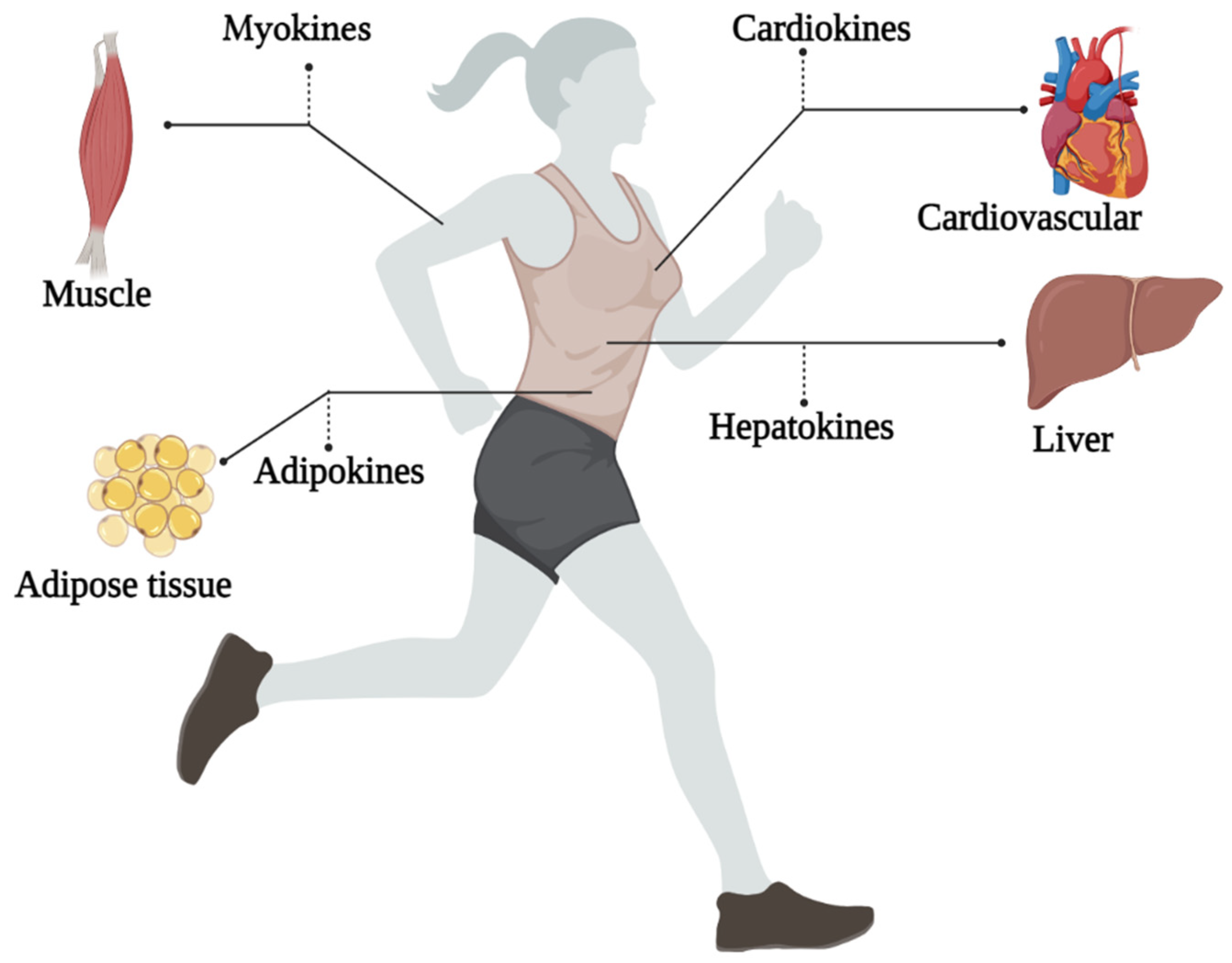
Figure 1. An overview of the exerkines investigated.
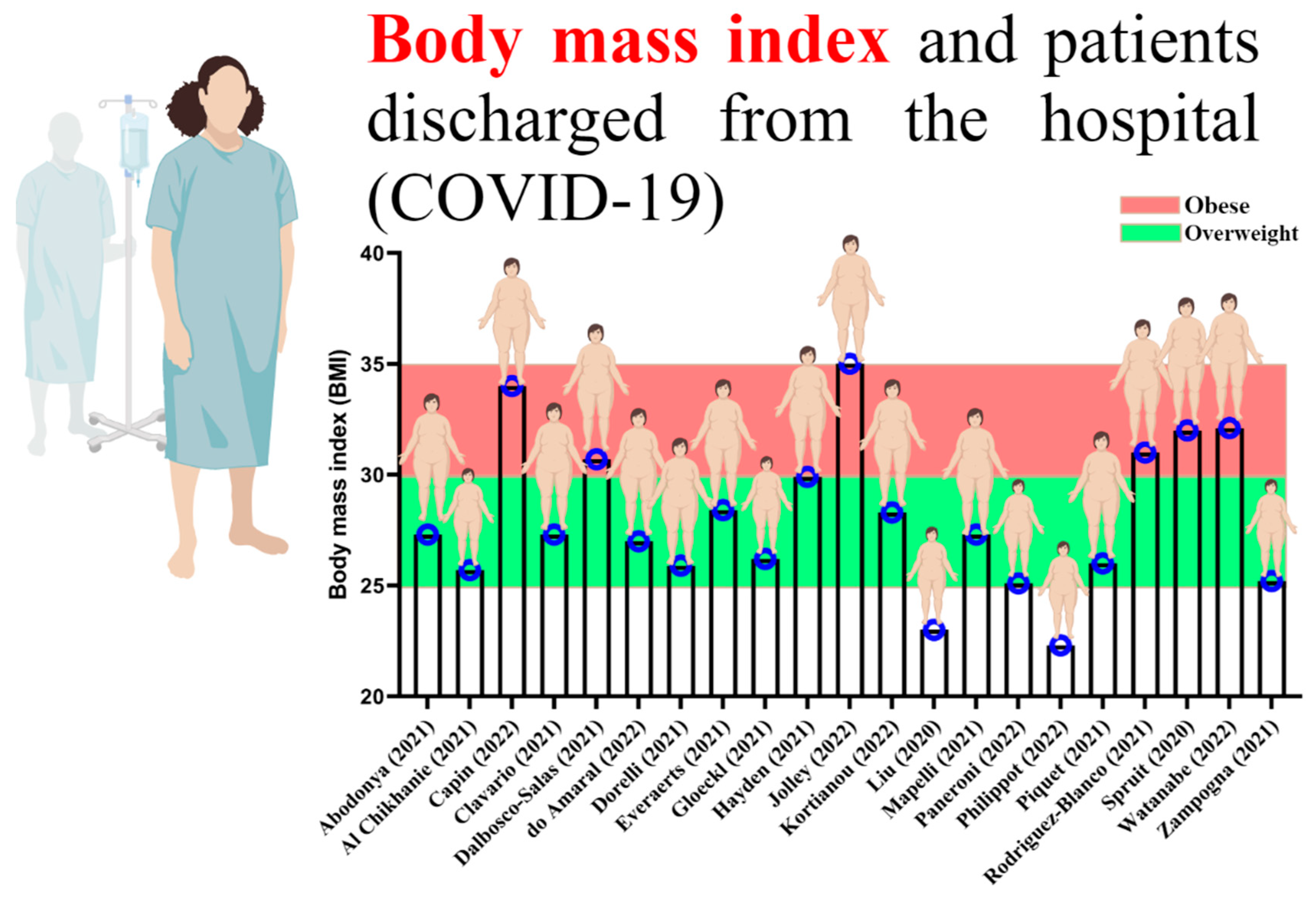
By reviewing past studies related to rehabilitation and physical exercise in the survivors of COVID-19, it can be well understood that most of those who were infected with COVID-19 were overweight and obese (Figure 2) [37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56].
2. The Potential Role of Exerkines Can Be Effective in COVID-19
It is well known that the most affected organs in COVID-19 include fat tissue, lungs, muscles, liver, and heart [57]. With the binding of SARS-CoV-2 spike protein to ACE2, physiological changes are created in the tissue, which can increase the disease process and influence the severity of the disease [58]. Moreover, the side effects of COVID-19 can stay with the patient long after discharge from the hospital and reduce the quality of life [59]. In this regard, most sports studies implementing “rehabilitation exercises” after COVID-19 had a general view of the effect of rehabilitation exercises on patients discharged from the hospital and did not investigate the fundamental mechanisms but highlighted the importance of physical exercise. Nevertheless, to provide a suitable physical exercise strategy for this disease, the mechanisms behind the curtain should be investigated. The idea of exerkines to take a more detailed look at diseases in 2022 by Chow et al. in the journal Nature Reviews Endocrinology gave rise to the idea that it might be possible to investigate the mechanisms behind the curtain and the effect of physical exercise in COVID-19 [27].
Exerkines look at the effect of physical exercise acutely or chronically on disease [27], and frequency, intensity, time, and type (FITT) can help to understand the effect of exerkines on disease better [27]. Exerkines are secreted in response to acute exercise (short-term physical exercise and less than 2 weeks), which is usually a part of aerobic or resistance training. Chronic exercise (long-term physical exercise and more than 2 weeks) is associated with changes in humoral factors, even at rest, suggesting that exerkine changes may reflect the effects of chronic exercise [60]. The acute exerkine response is influenced by the type of physical exercise, duration of physical exercise, background fitness, feeding–fasting status, and post-exercise sampling time [61]. Classical exerkines released during acute exercise, as found in human and animal models, include IL-6, IL-8, IL-1 receptor antagonist (IL-1ra), and IL-10. In a human study in which blood samples were collected before and after a marathon race (acute exercise), plasma levels of several cytokines (IL-6, IL-1ra, IL-10, and tumor necrosis factor (TNF)), when collected immediately, were higher than the base. Plasma levels measured after physical exercise were found to peak and remain high for up to 4 h after exercise [62]. Notably, the acute exerkine response does not necessarily parallel the chronic exerkine response [63].
This entry is adapted from the peer-reviewed paper 10.3390/ijerph192315645
References
- Schett, G.; Manger, B.; Simon, D.; Caporali, R. COVID-19 revisiting inflammatory pathways of arthritis. Nat. Rev. Rheumatol. 2020, 16, 465–470.
- Zhang, W.; Chua, B.Y.; Selva, K.J.; Kedzierski, L.; Ashhurst, T.M.; Haycroft, E.R.; Shoffner-Beck, S.K.; Hensen, L.; Boyd, D.F.; James, F.; et al. SARS-CoV-2 infection results in immune responses in the respiratory tract and peripheral blood that suggest mechanisms of disease severity. Nat. Commun. 2022, 13, 2774.
- Yu, W.; Rohli, K.E.; Yang, S.; Jia, P. Impact of obesity on COVID-19 patients. J. Diabetes Complicat. 2021, 35, 107817.
- Steenblock, C.; Hassanein, M.; Khan, E.G.; Yaman, M.; Kamel, M.; Barbir, M.; Lorke, D.E.; Everett, D.; Bejtullah, S.; Lohmann, T. Obesity and COVID-19: What are the consequences? Horm. Metab. Res. 2022, 54, 496–502.
- Cai, Q.; Chen, F.; Wang, T.; Luo, F.; Liu, X.; Wu, Q.; He, Q.; Wang, Z.; Liu, Y.; Liu, L. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 2020, 43, 1392–1398.
- Soares, M.N.; Eggelbusch, M.; Naddaf, E.; Gerrits, K.H.; van der Schaaf, M.; van den Borst, B.; Wiersinga, W.J.; van Vugt, M.; Weijs, P.J.; Murray, A.J. Skeletal muscle alterations in patients with acute Covid-19 and post-acute sequelae of Covid-19. J. Cachexia Sarcopenia Muscle 2022, 13, 11–22.
- Moro, T.; Paoli, A. When COVID-19 affects muscle: Effects of quarantine in older adults. Eur. J. Transl. Myol. 2020, 30, 9069.
- Di Stefano, V.; Battaglia, G.; Giustino, V.; Gagliardo, A.; D’Aleo, M.; Giannini, O.; Palma, A.; Brighina, F. Significant reduction of physical activity in patients with neuromuscular disease during COVID-19 pandemic: The long-term consequences of quarantine. J. Neurol. 2021, 268, 20–26.
- Scully, E.P.; Haverfield, J.; Ursin, R.L.; Tannenbaum, C.; Klein, S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020, 20, 442–447.
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320.
- Takahashi, T.; Iwasaki, A. Sex differences in immune responses. Science 2021, 371, 347–348.
- Burki, T. The indirect impact of COVID-19 on women. Lancet Infect. Dis. 2020, 20, 904–905.
- WHO. Gender and COVID-19: Advocacy Brief, 14 May 2020; World Health Organization: Geneva, Switzerland, 2020.
- Boniol, M.; McIsaac, M.; Xu, L.; Wuliji, T.; Diallo, K.; Campbell, J. Gender Equity in the Health Workforce: Analysis of 104 Countries; World Health Organization: Geneva, Switzerland, 2019.
- Connor, J.; Madhavan, S.; Mokashi, M.; Amanuel, H.; Johnson, N.R.; Pace, L.E.; Bartz, D. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: A review. Soc. Sci. Med. 2020, 266, 113364.
- Ahmed, S.B.; Dumanski, S.M. Sex, gender and COVID-19: A call to action. Can. J. Public Health 2020, 111, 980–983.
- United Nations. Policy Brief: The Impact of COVID-19 on Women—9 April 2020. 2020. Available online: https://unsdg.un.org/resources/policy-brief-impact-covid-19-women (accessed on 3 June 2022).
- Rubin, R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA 2020, 324, 1381–1383.
- Cooper, A.J.; Gupta, S.R.; Moustafa, A.F.; Chao, A.M. Sex/Gender Differences in Obesity Prevalence, Comorbidities, and Treatment. Curr. Obes. Rep. 2021, 10, 458–466.
- Leblanc, V.; Hudon, A.M.; Royer, M.M.; Corneau, L.; Dodin, S.; Bégin, C.; Lemieux, S. Differences between men and women in dietary intakes and metabolic profile in response to a 12-week nutritional intervention promoting the Mediterranean diet. J. Nutr. Sci. 2015, 4, e13.
- Jacobsen, H.; Klein, S.L. Sex Differences in Immunity to Viral Infections. Front. Immunol. 2021, 12, 720952.
- Stelzig, K.E.; Canepa-Escaro, F.; Schiliro, M.; Berdnikovs, S.; Prakash, Y.; Chiarella, S.E. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1280–L1281.
- Gupte, M.; Thatcher, S.E.; Boustany-Kari, C.M.; Shoemaker, R.; Yiannikouris, F.; Zhang, X.; Karounos, M.; Cassis, L.A. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1392–1399.
- Fishman, J.; Boyar, R.M.; Hellman, L. Influence of body weight on estradiol metabolism in young women. J. Clin. Endocrinol. Metab. 1975, 41, 989–991.
- Khoramipour, K.; Basereh, A.; Hekmatikar, A.A.; Castell, L.; Ruhee, R.T.; Suzuki, K. Physical activity and nutrition guidelines to help with the fight against COVID-19. J. Sports Sci. 2021, 39, 101–107.
- Shariat, A.; Cleland, J.A.; Hakakzadeh, A. Home-based exercises during the COVID-19 quarantine situation for office workers: A commentary. Work 2020, 66, 381–382.
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289.
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223.
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028.
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131.
- Ahmadi Hekmatikar, A.H.; Ferreira Júnior, J.B.; Shahrbanian, S.; Suzuki, K. Functional and Psychological Changes after Exercise Training in Post-COVID-19 Patients Discharged from the Hospital: A PRISMA-Compliant Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2290.
- Khoramipour, K.; Hekmatikar, A.A.; Sotvan, H. An overview of Fatmax and MFO in exercise. Razi J. Med. Sci. 2020, 27, 49–59.
- Tayebi, S.M.; Hekmatikar, A.A.; Ghanbari-Niaki, A.; Fathi, R. Ghrelin behavior in exercise and training. J Med. Sci 2020, 27, 85–111.
- Ahmadi Hekmatikar, A.; Haghshenas, R.; Mohammad Sadeghipor, A. The effect of carbohydrate supplementation and pure water on interleukin 10, glucose and hematologicalindexes in male football players. Sport Physiol. Manag. Investig. 2019, 11, 135–145.
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462.
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517.
- Abodonya, A.M.; Abdelbasset, W.K.; Awad, E.A.; Elalfy, I.E.; Salem, H.A.; Elsayed, S.H. Inspiratory muscle training for recovered COVID-19 patients after weaning from mechanical ventilation: A pilot control clinical study. Medicine 2021, 100, e25339.
- Al Chikhanie, Y.; Veale, D.; Schoeffler, M.; Pépin, J.L.; Verges, S.; Hérengt, F. Effectiveness of pulmonary rehabilitation in COVID-19 respiratory failure patients post-ICU. Respir. Physiol. Neurobiol. 2021, 287, 103639.
- Capin, J.J.; Jolley, S.E.; Morrow, M.; Connors, M.; Hare, K.; MaWhinney, S.; Nordon-Craft, A.; Rauzi, M.; Flynn, S.; Stevens-Lapsley, J.E.; et al. Safety, feasibility and initial efficacy of an app-facilitated telerehabilitation (AFTER) programme for COVID-19 survivors: A pilot randomised study. BMJ Open 2022, 12, e061285.
- Clavario, P.; De Marzo, V.; Lotti, R.; Barbara, C.; Porcile, A.; Russo, C.; Beccaria, F.; Bonavia, M.; Bottaro, L.C.; Caltabellotta, M. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int. J. Cardiol. 2021, 340, 113–118.
- Dalbosco-Salas, M.; Torres-Castro, R.; Rojas Leyton, A.; Morales Zapata, F.; Henríquez Salazar, E.; Espinoza Bastías, G.; Beltrán Díaz, M.E.; Tapia Allers, K.; Mornhinweg Fonseca, D.; Vilaró, J. Effectiveness of a Primary Care Telerehabilitation Program for Post-COVID-19 Patients: A Feasibility Study. J. Clin. Med. 2021, 10, 4428.
- do Amaral, V.T.; Viana, A.A.; Heubel, A.D.; Linares, S.N.; Martinelli, B.; Witzler, P.H.C.; de Souza Zanini, G.; Mendes, R.; Ciolac, E. Cardiovascular, Respiratory, and Functional Effects of Home-based Exercise Training after COVID-19 Hospitalization. Med. Sci. Sports Exerc. 2022, 54, 1795–1803.
- Dorelli, G.; Braggio, M.; Gabbiani, D.; Busti, F.; Caminati, M.; Senna, G.; Girelli, D.; Laveneziana, P.; Ferrari, M.; Sartori, G.; et al. Importance of Cardiopulmonary Exercise Testing amongst Subjects Recovering from COVID-19. Diagnostics 2021, 11, 507.
- Everaerts, S.; Heyns, A.; Langer, D.; Beyens, H.; Hermans, G.; Troosters, T.; Gosselink, R.; Lorent, N.; Janssens, W. COVID-19 recovery: Benefits of multidisciplinary respiratory rehabilitation. BMJ Open Respir. Res. 2021, 8, e000837.
- Gloeckl, R.; Leitl, D.; Jarosch, I.; Schneeberger, T.; Nell, C.; Stenzel, N.; Vogelmeier, C.F.; Kenn, K.; Koczulla, A.R. Benefits of pulmonary rehabilitation in COVID-19: A prospective observational cohort study. ERJ Open Res. 2021, 7, 00108–02021.
- Hayden, M.C.; Limbach, M.; Schuler, M.; Merkl, S.; Schwarzl, G.; Jakab, K.; Nowak, D.; Schultz, K. Effectiveness of a three-week inpatient pulmonary rehabilitation program for patients after COVID-19: A prospective observational study. Int. J. Environ. Res. Public Health 2021, 18, 9001.
- Kortianou, E.A.; Tsimouris, D.; Mavronasou, A.; Lekkas, S.; Kazatzis, N.; Apostolara, Z.E.; Isakoglou, M.; Dimakou, G.; Barmparessou, Z.; Tsikrika, S. Application of a home-based exercise program combined with tele-rehabilitation in previously hospitalized patients with COVID-19: A feasibility, single-cohort interventional study. Pneumon 2022, 35, 12.
- Liu, K.; Zhang, W.; Yang, Y.; Zhang, J.; Li, Y.; Chen, Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 2020, 39, 101166.
- Mapelli, M.; Vignati, C.; Gugliandolo, P.; Fumagalli, D.; Agostoni, P. Feasibility of remote home monitoring with a T-shirt wearable device in post-recovery COVID-19 patients. J. Cardiovasc. Med. 2021, 22, 860–863.
- Paneroni, M.; Vitacca, M.; Bernocchi, P.; Bertacchini, L.; Scalvini, S. Feasibility of tele-rehabilitation in survivors of COVID-19 pneumonia. Pulmonology 2022, 28, 152.
- Philippot, A.; Moulin, P.; Charon, M.-H.; Balestra, C.; Dubois, V.; de Timary, P.; De Volder, A.; Bleyenheuft, Y.; Lambrechts, K. Feasibility of Online High-Intensity Interval Training (HIIT) on Psychological Symptoms in Students in Lockdown During the COVID-19 Pandemic: A Randomized Controlled Trial. Front. Psychiatry 2022, 13, 904283.
- Piquet, V.; Luczak, C.; Seiler, F.; Monaury, J.; Martini, A.; Ward, A.B.; Gracies, J.M.; Motavasseli, D. Do Patients With COVID-19 Benefit from Rehabilitation? Functional Outcomes of the First 100 Patients in a COVID-19 Rehabilitation Unit. Arch. Phys. Med. Rehabil. 2021, 102, 1067–1074.
- Rodriguez-Blanco, C.; Gonzalez-Gerez, J.J.; Bernal-Utrera, C.; Anarte-Lazo, E.; Perez-Ale, M.; Saavedra-Hernandez, M. Short-Term Effects of a Conditioning Telerehabilitation Program in Confined Patients Affected by COVID-19 in the Acute Phase. A Pilot Randomized Controlled Trial. Medicina 2021, 57, 684.
- Spruit, M.A.; Holland, A.E.; Singh, S.J.; Tonia, T.; Wilson, K.C.; Troosters, T. COVID-19: Interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society-and American Thoracic Society-coordinated international task force. Eur. Respir. J. 2020, 56, 2002197.
- Watanabe, R.; Kojima, M.; Yasuoka, M.; Kimura, C.; Kamiji, K.; Otani, T.; Tsujimura, S.; Fujita, H.; Nogimura, A.; Ozeki, S. Home-Based Frailty Prevention Program for Older Women Participants of Kayoi-No-Ba during the COVID-19 Pandemic: A Feasibility Study. Int. J. Environ. Res. Public Health 2022, 19, 6609.
- Zampogna, E.; Paneroni, M.; Belli, S.; Aliani, M.; Gandolfo, A.; Visca, D.; Bellanti, M.T.; Ambrosino, N.; Vitacca, M. Pulmonary rehabilitation in patients recovering from COVID-19. Respiration 2021, 100, 416–422.
- Peiris, S.; Mesa, H.; Aysola, A.; Manivel, J.; Toledo, J.; Borges-Sa, M.; Aldighieri, S.; Reveiz, L. Pathological findings in organs and tissues of patients with COVID-19: A systematic review. PLoS ONE 2021, 16, e0250708.
- Bhalla, V.; Blish, C.A.; South, A.M. A historical perspective on ACE2 in the COVID-19 era. J. Hum. Hypertens. 2021, 35, 935–939.
- Zarei, M.; Bose, D.; Nouri-Vaskeh, M.; Tajiknia, V.; Zand, R.; Ghasemi, M. Long-term side effects and lingering symptoms post COVID-19 recovery. Rev. Med. Virol. 2022, 32, e2289.
- Barisic, A.; Leatherdale, S.T.; Kreiger, N. Importance of frequency, intensity, time and type (FITT) in physical activity assessment for epidemiological research. Can. J. Public Health 2011, 102, 174–175.
- Leuchtmann, A.B.; Adak, V.; Dilbaz, S.; Handschin, C. The role of the skeletal muscle secretome in mediating endurance and resistance training adaptations. Front. Physiol. 2021, 12, 1296.
- Wahren, J.; Felig, P.; Ahlborg, G.; Jorfeldt, L. Glucose metabolism during leg exercise in man. J. Clin. Investig. 1971, 50, 2715–2725.
- Magliulo, L.; Bondi, D.; Pini, N.; Marramiero, L.; Di Filippo, E.S. The wonder exerkines—Novel insights: A critical state-of-the-art review. Mol. Cell. Biochem. 2021, 447, 105–113.
This entry is offline, you can click here to edit this entry!