Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Civil
Alkali-activated materials (AAM) are recognized as potential alternatives to ordinary Portland cement (OPC) to limit CO2 emissions and beneficiate several wastes into useful products. Compared with its counterparts involving the concentrated aqueous alkali solutions, the development of “just add water” one-part alkali-activated materials (OP-AAM) has drawn much attention, mainly attributed to their benefits in overcoming the hazardous, irritating, and corrosive nature of activator solutions.
- alkali-activated
- one-part
- geopolymer
- mix design
- mechanical properties
- durability
1. Workability
The fresh performance of the OP-AAM is mainly evaluated by the slump and setting time. As depicted in Figure 7, the alkali–binder ratio (a/b ratio), water–binder ratio (w/b ratio), and GGBS content do affect the workability of the AAM. The most sensitive parameter is the w/b ratio. With the increase in water consumption, both the slump and setting time are enhanced with the reduced mechanical properties. It can also be found from Figure 7 that the hardening properties of the paste are mainly affected by the value of the a/b ratio. Although the setting time of the AAM is further reduced as the alkali content increases, the increased a/b ratio will affect the flowability. The gels of polymeric products depend on the dissolution of precursors. In a highly alkaline environment, Si and Al are dissolved and polymerized to form hydraulic binders [119]. Increasing the alkali content will accelerate this process [120]. Different from the positive results reported by lots of scholars, Teo et al. [44] and Li et al. [64] found a rapid decrease in slump when the alkali equivalent exceeded a certain limit.
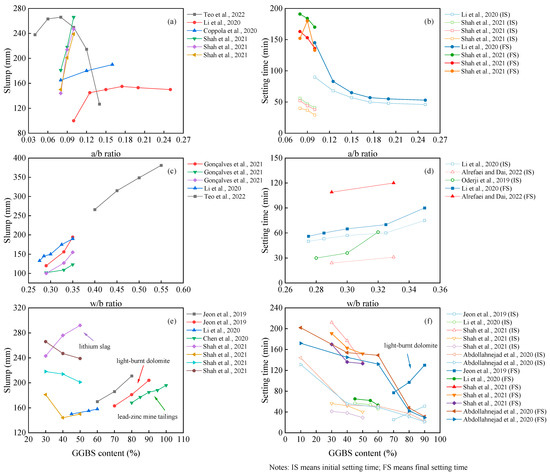
As mentioned before, many materials can be incorporated into the mix design, and their performance varies. In this section, we take the slag, for example, in comparing the workability of the OP-AAM with different slag replacements. It can be seen that the properties of the AAM are different when the slag is added to different precursors. Most research showed that the slag-based AAM obtains fast hardening properties, resulting in a decrease in slump and setting time at a higher slag replacement [121]. As listed in Figure 7, the slump tends to increase with higher slag content, while the setting time is consistent with the conventional pattern. The possible reason for this finding may be attributed to the higher reactivity of the slag when light-burnt dolomite (LBD) is used as a precursor. The active Ca and Mg components in LBD generate calcium hydroxide, C-A-S-H gels, and magnesium hydroxide, which will affect the reaction heat of the slag-based system [122]. In contrast, Chen et al. [91] using lead–zinc mine tailings and Shah et al. [54] using lithium slag blended into a slag-based AAM found that higher fineness needs higher water requirement; thus, the slump loss is due to the fineness, not the reactivity [123].
In addition, the evaluation of workability has different parameters specified in different standards, such as the use of European standards EN 1015-3 and EN196-3 [80] and Indian standard IS 1199–1959 [124]. These standards use a minislump test to characterize workability, which cannot be evaluated uniformly with the above index and is not listed to facilitate the comparison of similar parameters.
2. Rheology
The material resistance to flow after the material begins to flow and the shear stress required to initiate flow, namely, plastic viscosity and yield stress, respectively, are important parameters in the rheological properties of the AAM [125]. For the one-part AAM, when the water content is determined, increasing the dosage of the activator may reduce the yield stress [126]. Owing to the influence of ionic equilibrium in the dissolution process of the solid activator, the dissolution rate becomes slow when the solution is close to saturation, and the equilibrium shear stress is reduced. Generally speaking, the rheological law of the OP-AAM is similar to that of the traditional AAM. The basicity of the solid activator after dissolution affects the dissolution efficiency of the precursor, and the alkali cation mainly plays the role of charge balance [120]. Among the rheology of the traditional AAM, the sodium silicate activator may have a lower yield stress, which is due to silicate oligomers adsorbing on the surfaces of slag particles, resulting in insufficient dissolved Ca2+ in the solution and greater repulsive double-layer forces [126]. However, when sodium silicate is added to a solid phase, the yield stress and plastic viscosity could be increased [127]
In addition to the activator, aluminosilicate also has a great influence on rheological behavior. Different from GGBS, it dissolves faster and has higher reactivity in alkaline environments [128,129]. The “ball-bearings” and slow dissolution rate of FA make gel precipitate slower at the initial stage of the reaction [129], reducing the yield stress of pastes and affecting its rheological [130,131]. On the contrary, Alrefaei et al. concluded that the addition of FA can increase the yield stress of the OP-AAM, which may be related to the high porosity and surface area of carbon-containing impurities [126]. MK generally exhibits high viscosity and yield stress, probably due to the platelike particle shape and high surface area [119,132]. It means that a suitable activator and enough water are needed to ensure acceptable performance [132,133].
3. Compressive Strength
The application of the slag can provide the highest compressive strength of all mix designs [48,71]. This is mainly due to its calcium content and the C-S-H gel generated by the hydration reaction, the latter of which greatly improves the mechanical properties [81]. The incorporation of other precursors in slag-based AAM can retard the setting time [41,64,108] and reduce the drying shrinkage [61,101,134], which can meet the workability requirement. Under specific conditions (e.g., high curing temperature, chemical modification), the fly ash or metakaolin can also achieve satisfactory mechanical properties [71,91,102], providing a variable precursor to be selected. In terms of alkali sources, sodium silicate is most effective, followed by sodium hydroxide [67,73]. The performance of other alkali sources, such as carbonates [47], aluminates [71,94], calcium oxides [21,77], and potassium oxides [90,134], has been successfully used for preparation. When these materials are blended with water, the alkali environment as well as aluminum and silicon elements for the hydration reaction may be generated.
It can be seen from Figure 8 that the compressive strength gradually increases with the values of the a/b ratio and GGBS content. The possible reason for this observation is that the increased a/b ratio within a certain range (generally in the range of 5–12%) can promote the hydration reaction. Meanwhile, there is a risk of efflorescence on the surface [79], forming pores and reducing the compactness [135] when it is at high concentrations. The effect of the w/b ratio on the compressive strength of the AAM is negative, which agrees well with previous research on other cementitious materials.
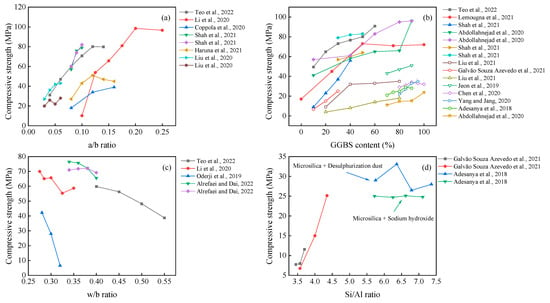
The effect of the Si/Al ratio can also be seen in Figure 8. Increasing the SiO2/Al2O3 molar ratio positively contributes to compressive strength and modulus of elasticity, together with a decreased porosity [136]. Meanwhile, the unreacted materials decrease with increased alkali and silicon content [59]. In contrast, Adesanya et al. [20] found that the use of silica fume to increase the soluble silica along with desulfurization dust (DeS) resulted in better mechanical properties than sodium hydroxide. However, this pattern was not monotonically increasing, reaching up to 33.6 MPa at Si/Al = 6.36. This induced change in mechanical properties could be attributed to the fact that Fe or Ca elements for additional dissolution and the beneficial effect of Fe in AAM have been demonstrated by Simon et al. and Adesanya et al. [137,138].
If only superior mechanical properties are requested, using highly reactive materials and improving the a/b ratio and Si/Al ratio are believed to be effective methods. However, the fact is that the fine-grained materials usually require much more water to meet the workability, and the higher w/b ratio brings a side-effect on the mechanical response. That is, developing an effective superplasticizer for the AAM becomes critical in balancing the mechanical and physical properties.
4. Durability
The durability of the AAM prepared by different precursors is different. Although the AAM exhibits superior resistance to carbonation and sulfate attack, the freezing resistance of which has not been well evaluated. Alzaza et al. [55] found that the incomplete hydration and internal deterioration led to significantly lower freezing resistance when it was curved at −20 °C. As a result, increasing the curing temperature is beneficial to form a dense microstructure and improve the freezing resistance. Coppola et al. [90] considered the effect of the durability of the slag-based AAM under the influence of an air-entraining agent (AEA), the addition of AEA improved the freezing resistance of all tests, the test groups with high alkalinity (AAS12, AAS16) could achieve freezing resistance similar to that of OPC, and slag-based AAM showed higher corrosion resistance to the CaCl2 condition than the MgSO4 condition, which can be attributed to the decalcification of the C-S-H gel and the formation of the expansion product gypsum. Ahmad et al. [92] investigated the performance of a GGBS/FA-based AAM against sulfate and acid corrosion under different mineral admixtures. All their samples were subjected to up to 270 d of age immersion in sodium sulfate solution with only up to 17% loss of strength. It has been found that there is better resistance to sulfate attack (1% loss of strength) at 4.5% metakaolin replacement, while samples in a sulfuric acid environment showed varying degrees of cracking and spalling, which was also observed in acid resistance experiments by Sturm et al. [94].
Existing studies on the durability of the OP-AAM are extremely limited, and it appears that slag-based materials may deteriorate in performance under sulfate attacking due to expansive products, which is an obstacle to be overcome. Excellent resistance to carbonation and high temperatures has been demonstrated by several studies. However, the deficiency of freezing resistance is not good enough, which is similar to the conventional AAM [17,139]. This does not prove whether there is a performance difference in the AAM prepared by traditional alkali solutions. As a result, more studies are requested to clarify both the similarities and differences of the AAM with two typical preparation techniques. The investigation results will be extremely important to better understand the service performance of the AAM in harsh environments.
This entry is adapted from the peer-reviewed paper 10.3390/polym14225046
This entry is offline, you can click here to edit this entry!
