Additive manufacturing, also called 3D printing, is the process of building up a three-dimensional (3D) object from computer-aided-design (CAD) models through a layer-by-layer fabrication process. Polyolefins are semi-crystalline thermoplastic polymers known for their good mechanical properties, low production cost, and chemical resistance. They are amongst the most commonly used plastics, and many polyolefin grades are regarded as engineering polymers. The two main additive manufacturing techniques that can be used to fabricate 3D-printed parts are fused filament fabrication and selective laser sintering. Polyolefins, like polypropylene and polyethylene, can, in principle, be processed with both these techniques. The semi-crystalline nature of polyolefins adds complexity to the use of additive manufacturing methods compared to amorphous polymers.
- polyolefins
- polyethylene
- 3D
1. Polypropylene
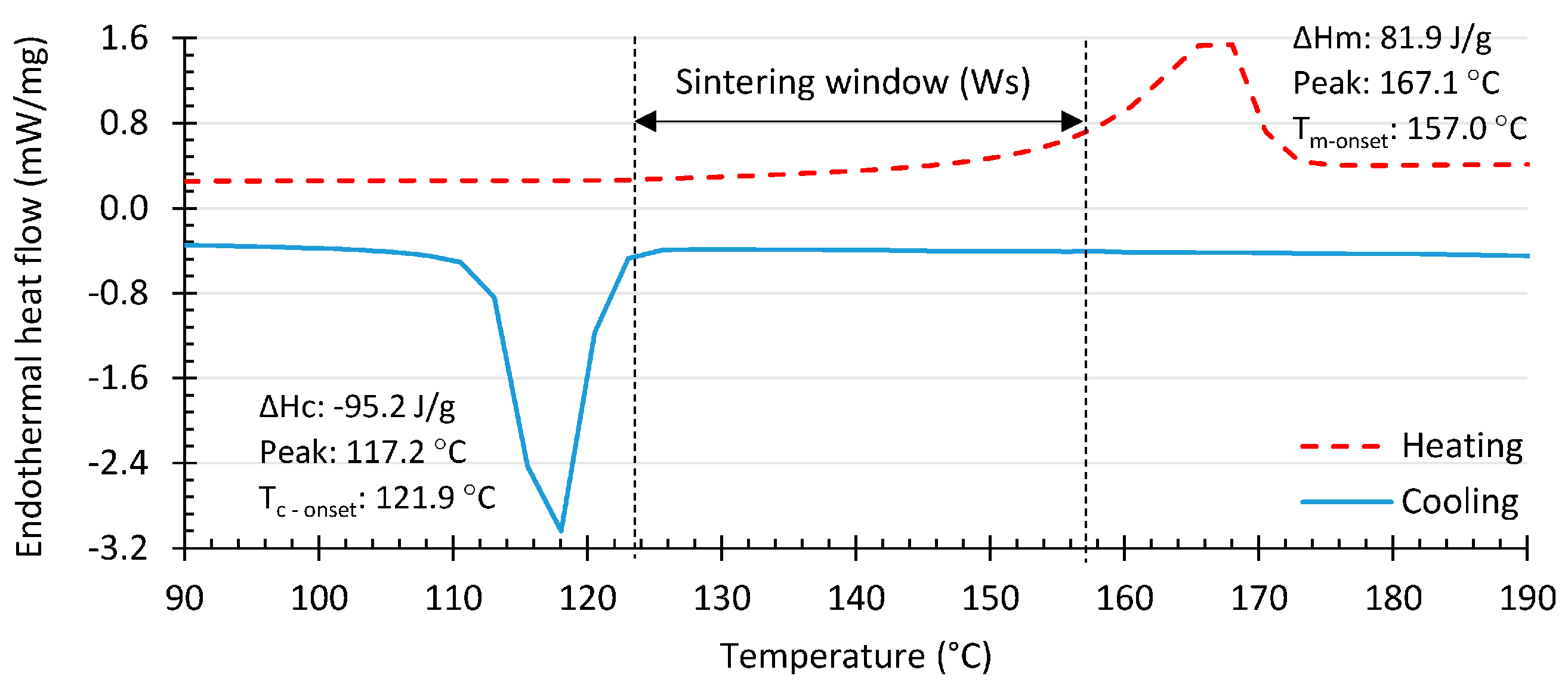
2. Low- and High-Density Polyethylene
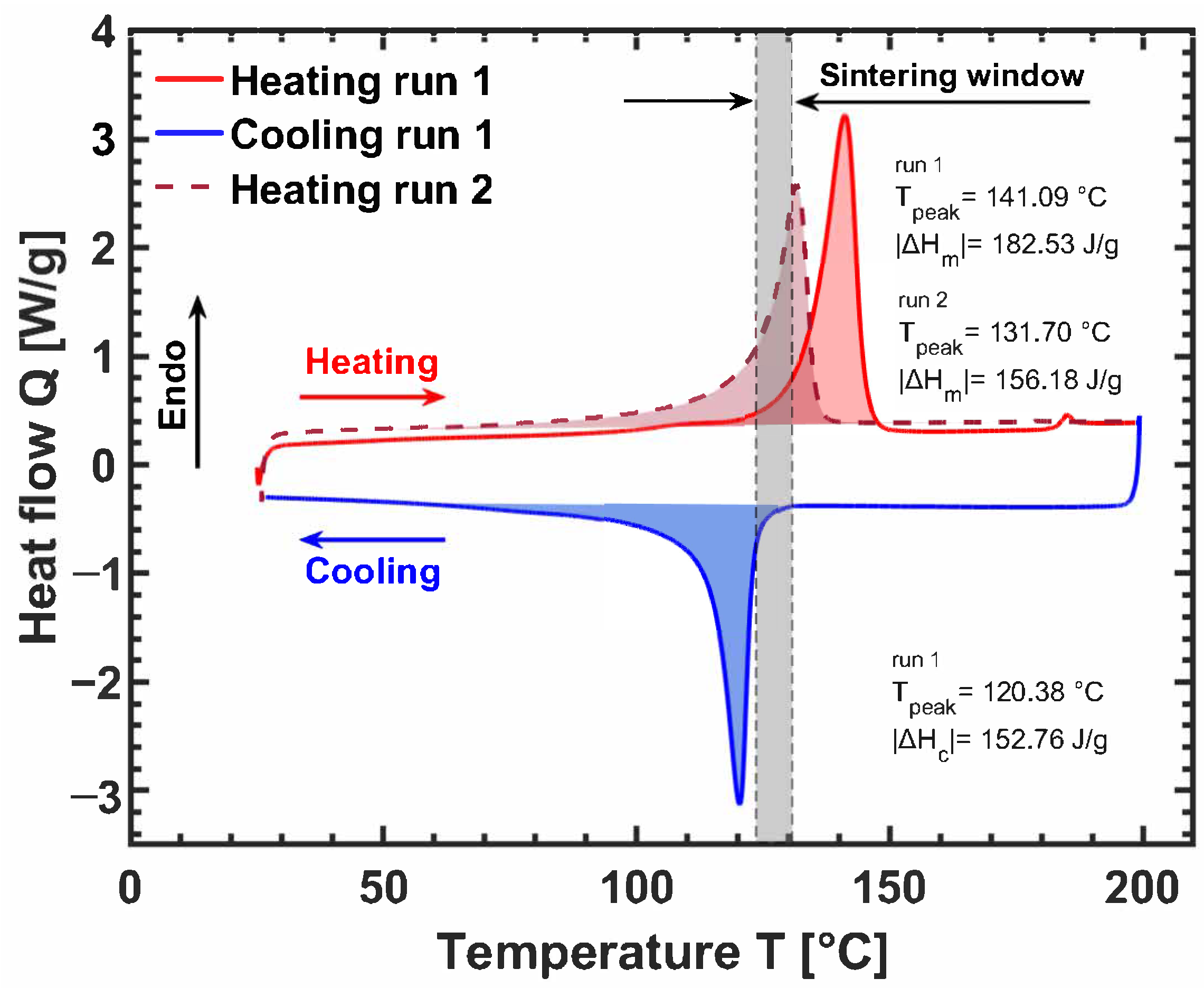
3. Ultra-High Molecular Weight Polyethylene
This entry is adapted from the peer-reviewed paper 10.3390/polym14235147
References
- Carneiro, O.S.; Silva, A.F.; Gomes, R. Fused deposition modeling with polypropylene. Mater. Des. 2015, 83, 768–776.
- Caelers, H.J.; Troisi, E.M.; Govaert, L.E.; Peters, G.W. Deformation-induced phase transitions in iPP polymorphs. Polymers 2017, 9, 547.
- Varga, J. β-modification of isotactic polypropylene: Preparation, structure, processing, properties, and application. J. Macromol. Sci. Part B 2002, 41, 1121–1171.
- Lotz, B.; Wittmann, J.C.; Lovinger, A.J. Structure and morphology of poly(propylenes): A molecular analysis. Polymer 1996, 37, 4979–4992.
- Brückner, S.; Meille, S.V.; Petraccone, V.; Pirozzi, B. Polymorphism in isotactic polypropylene. Prog. Polym. Sci. 1991, 16, 361–404.
- Jones, A.T.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Die Makromol. Chem. 1964, 75, 134.
- Keith, H.D.; Padden, F.J.; Walter, N.M.; Wyckoff, H.W. Evidence for a second crystal form of polypropylene. J. Appl. Phys. 1959, 30, 1485–1488.
- Addink, E.J.; Beintema, J. Polymorphism of crystalline polypropylene. Polymer 1961, 2, 185–193.
- Silva, A.F.; Carneiro, O.S.; Gomes, R. 3D printing of polypropylene using the fused filament fabrication technique. AIP Conf. Proc. 2017, 1896, 040014.
- Charlon, S.; Le Boterff, J.; Soulestin, J. Fused filament fabrication of polypropylene: Influence of the bead temperature on adhesion and porosity. Addit. Manuf. 2021, 38, 101838.
- Wang, L.; Sanders, J.E.; Gardner, D.J.; Han, Y. Effect of fused deposition modeling process parameters on the mechanical properties of a filled polypropylene. Prog. Addit. Manuf. 2018, 3, 205–214.
- Davis, C.S.; Hillgartner, K.E.; Han, S.H.; Seppala, J.E. Mechanical strength of welding zones produced by polymer extrusion additive manufacturing. Addit. Manuf. 2017, 16, 162–166.
- van Erp, T.B.; Balzano, L.; Spoelstra, A.B.; Govaert, L.E.; Peters, G.W.M. Quantification of non-isothermal, multiphase crystallization of isotactic polypropylene: The influence of shear and pressure. Polymer 2012, 53, 5896–5908.
- Tordjeman, P.; Robert, C.; Marin, G.; Gerard, P. The effect of α, β crystalline structure on the mechanical properties of polypropylene. Eur. Phys. J. E 2001, 4, 459–465.
- Fillon, B.; Thierry, A.; Wittmann, J.C.; Lotz, B. Self-nucleation and recrystallization of polymers. Isotactic polypropylene, β phase: β-α conversion and β-α growth transitions. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1407–1424.
- Vaes, D.; Van Puyvelde, P. Semi-crystalline feedstock for filament-based 3D printing of polymers. Prog. Polym. Sci. 2021, 118, 101411.
- Northcutt, L.A.; Orski, S.V.; Migler, K.B.; Kotula, A.P. Effect of processing conditions on crystallization kinetics during materials extrusion additive manufacturing. Polymer 2018, 154, 182–187.
- Eder, G.; Janeschitz-Kriegl, H.; Krobath, G. Shear induced crystallization, a relaxation phenomenon in polymer melts. Relax. Polym. 1989, 80, 1–7.
- Janeschitz-Kriegl, H.; Eder, G. Shear induced crystallization, a relaxation phenomenon in polymer melts: A re-collection. J. Macromol. Sci. Part B Phys. 2007, 46, 591–601.
- Ituarte, I.F.; Wiikinkoski, O.; Jansson, A. Additive manufacturing of polypropylene: A screening design of experiment using laser-based powder bed fusion. Polymers 2018, 10, 1293.
- Zhu, W.; Yan, C.; Shi, Y.; Wen, S.; Liu, J.; Shi, Y. Investigation into mechanical and microstructural properties of polypropylene manufactured by selective laser sintering in comparison with injection molding counterparts. Mater. Des. 2015, 82, 37–45.
- Tan, L.J.; Zhu, W.; Sagar, K.; Zhou, K. Comparative study on the selective laser sintering of polypropylene homopolymer and copolymer: Processability, crystallization kinetics, crystal phases and mechanical properties. Addit. Manuf. 2021, 37, 101610.
- Valdo Meille, S.; Brückner, S. Non-parallel chains in crystalline γ-isotactic polypropylene. Nature 1989, 340, 455–457.
- Mezghani, K.; Phillips, P.J. The γ-phase of high molecular weight isotactic polypropylene: III. The equilibrium melting point and the phase diagram. Polymer 1998, 39, 3735–3744.
- Lezak, E.; Bartczak, Z. Plastic deformation of the γ phase isotactic polypropylene in plane-strain compression at elevated temperatures. Macromolecules 2007, 40, 4933–4941.
- Kruth, J.; Levy, G.; Schindel, R.; Craeghs, T.; Yasa, E. Consolidation of Polymer Powders by Selective Laser Sintering. In Proceedings of the International Conference on Polymers and Moulds Innovations, Gent, Belgium, 17–19 September 2008; pp. 15–30.
- Khanam, P.N.; AlMaadeed, M.A.A. Processing and characterization of polyethylene-based composites. Adv. Manuf. Polym. Compos. Sci. 2015, 1, 63–79.
- Jordan, J.L.; Casem, D.T.; Bradley, J.M.; Dwivedi, A.K.; Brown, E.N.; Jordan, C.W. Mechanical Properties of Low Density Polyethylene. J. Dyn. Behav. Mater. 2016, 2, 411–420.
- Olesik, P.; Godzierz, M.; Kozioł, M. Preliminary characterization of novel LDPE-based wear-resistant composite suitable for FDM 3D printing. Materials 2019, 12, 2520.
- Bedi, P.; Singh, R.; Ahuja, I.P. Effect of SiC/Al2O3 particle size reinforcement in recycled LDPE matrix on mechanical properties of FDM feed stock filament. Virtual Phys. Prototyp. 2018, 13, 246–254.
- Enderle, H. Polyethylene: High-density. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Subhash Mahajan, P.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Chapter 2; pp. 7172–7180.
- Mejia, E.B.; Al-Maqdi, S.; Alkaabi, M.; Alhammadi, A.; Alkaabi, M.; Cherupurakal, N.; Mourad, A.H.I. Upcycling of HDPE waste using additive manufacturing: Feasibility and challenges. In Proceedings of the 2020 Advances in Science and Engineering Technology International Conferences, Dubai, United Arab Emirates, 4 February–9 April 2020.
- Vidakis, N.; Petousis, M.; Maniadi, A. Sustainable additive manufacturing: Mechanical response of high-density polyethylene over multiple recycling processes. Recycling 2021, 6, 4.
- Schirmeister, C.G.; Hees, T.; Licht, E.H.; Mülhaupt, R. 3D printing of high density polyethylene by fused filament fabrication. Addit. Manuf. 2019, 28, 152–159.
- Salmoria, G.V.; Leite, J.L.; Paggi, R.A.; Lago, A.; Pires, A.T. Selective laser sintering of PA12/HDPE blends: Effect of components on elastic/plastic behavior. Polym. Test. 2008, 27, 654–659.
- Lisi Leite, J.; Salmoria, G.V.; Paggi, R.A.; Ahrens, C.H.; Pouzada, A.S. Microstructural characterization and mechanical properties of functionally graded PA12/HDPE parts by selective laser sintering. Int. J. Adv. Manuf. Technol. 2012, 59, 583–591.
- Salari, M.; Pircheraghi, G. Fabrication of sintered porous polymeric materials: Effect of chain interdiffusion time on mechanical properties. Polym. Int. 2018, 67, 422–430.
- Rajamani, D.; Balasubramanian, E. Investigation of sintering parameters on viscoelastic behaviour of selective heat sintered HDPE parts. J. Appl. Sci. Eng. 2019, 22, 39–402.
- Hejmady, P.; Van Breemen, L.C.; Anderson, P.D.; Cardinaels, R. Laser sintering of polymer particle pairs studied by in situ visualization. Soft Matter 2019, 15, 1373–1387.
- Barham, P.J.; Sadler, D.M. A neutron scattering study of the melting behaviour of polyethylene single crystals. Polymer 1991, 32, 393–395.
- Litvinov, V.; Deblieck, R.; Clair, C.; Van Den Fonteyne, W.; Lallam, A.; Kleppinger, R.; Ivanov, D.A.; Ries, M.E.; Boerakker, M. Molecular Structure, Phase Composition, Melting Behavior, and Chain Entanglements in the Amorphous Phase of High-Density Polyethylenes. Macromolecules 2020, 53, 5418–5433.
- Salehi, A.; Pircheraghi, G.; Foudazi, R. Pore structure evolution during sintering of HDPE particles. Polymer 2019, 183, 121865.
- Hoelzel, B.; Herren, B.; Saha, M.C.; Liu, Y. Investigation of selective laser sintering of high-density polyethylene using optimized 3D printing parameters. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Proceedings (IMECE), Virtual, 1–5 November 2021; Volume 4.
- Wencke, Y.L.; Proes, F.; Imgrund, P.; Luinstra, G.A. Toward the Direct Synthesis of HDPE Powders for Powder Bed Fusion Based Additive Manufacturing. Macromol. Mater. Eng. 2021, 306, 2100477.
- Chanzy, H.D.; Bonjour, E.; Marchessault, R.H. Nascent structures during the polymerization of ethylene—II. Calorimetric study. Colloid Polym. Sci. 1974, 252, 8–14.
- Wang, X.Y.; Salovey, R. Melting of ultrahigh molecular weight polyethylene. J. Appl. Polym. Sci. 1987, 34, 593–599.
- Christakopoulos, F.; Troisi, E.M.; Sologubenko, A.S.; Friederichs, N.; Stricker, L.; Tervoort, T.A. Melting kinetics, ultra-drawability and microstructure of nascent ultra-high molecular weight polyethylene powder. Polymer 2021, 222.
- Goodridge, R.D.; Hague, R.J.; Tuck, C.J. An empirical study into laser sintering of ultra-high molecular weight polyethylene (UHMWPE). J. Mater. Process. Technol. 2010, 210, 72–80.
- Khalil, Y.; Kowalski, A.; Hopkinson, N. Influence of laser power on tensile properties and material characteristics of laser-sintered UHMWPE. Manuf. Rev. 2016, 3, 15.
- Song, C.; Huang, A.; Yang, Y.; Xiao, Z.; Yu, J.K. Effect of energy input on the UHMWPE fabricating process by selective laser sintering. Rapid Prototyp. J. 2017, 23, 1069–1078.
- Ullsperger, T.; Wencke, Y.L.; Yürekli, B.; Matthäus, G.; Rettenmayr, M.; Luinstra, G.A.; Nolte, S. Laser powder bed fusion of ultra-high molecular weight polyethylene (UHMWPE) using near-infrared ultrashort laser pulses. Mater. Des. 2021, 210, 110048.
- Prager, S.; Tirrell, M. The healing process at polymer-polymer interfaces. J. Chem. Phys. 1981, 75, 5194–5198.
- Xue, Y.Q.; Tervoort, T.A.; Rastogi, S.; Lemstra, P.J. Welding behavior of semicrystalline polymers. 2. Effect of cocrystallization on autoadhesion. Macromolecules 2000, 33, 7084–7087.
- Wool, R.P.; Yuan, B.L.; McGarel, O.J. Welding of polymer interfaces. Polym. Eng. Sci. 1989, 29, 1340–1367.
- Wool, R.P. Self-healing materials: A review. Soft Matter 2008, 4, 400–418.
- De Gennes, P.G. The formation of polymer/polymer junctions. Tribol. Ser. 1981, 7, 355–367.
- Benkoski, J.J.; Fredrickson, G.H.; Kramer, E.J. Model for the fracture energy of glassy polymer-polymer interfaces. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2377–2386.
- Bastiaansen, C.W.; Meyer, H.E.; Lemstra, P.J. Memory effects in polyethylenes: Influence of processing and crystallization history. Polymer 1990, 31, 1435–1440.
- Gill, T.J.; Hon, K.K. Experimental investigation into the selective laser sintering of silicon carbide polyamide composites. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2004, 218, 1249–1256.
- Visco, A.M.; Torrisi, L.; Galtieri, G.; Scolaro, C. Effect of the filler amount on the optical absorption properties and the surface features of polymeric joints based on biomedical UHMWPE welded by a Nd:YAG laser. J. Thermoplast. Compos. Mater. 2017, 30, 1675–1692.
- Rimell, J.T.; Marquis, P.M. Selective laser sintering of ultra high molecular weight polyethylene for clinical applications. J. Biomed. Mater. Res. 2000, 53, 414–420.
