Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The overall 5-year survival rate of esophageal cancer patients is poor. Galectins are glycan-binding proteins known to contribute to tumor initiation and progression. To get insight in the expression and potential function of galectins in esophageal cancer, a literature review is performed. The researchers found that galectins have been mainly studied in the context of esophageal squamous cell carcinoma and that galectin-1, -3, and -9 expression are most frequently reported. More research is required to provide better insights in the diagnostic, prognostic, and predictive value of galectins in esophageal cancer as well as their functional role in tumor progression.
- squamous cell carcinoma
- adenocarcinoma
- gene expression
- protein expression
- diagnosis
- prognosis
- immunohistochemistry
1. The Galectin Protein Family
The galectin family consists of proteins that share a conserved carbohydrate-recognition domain (CRD) of approximately 130 amino acids which shows binding affinity for beta-galactosides [1]. The family name was coined in 1994 and at that time 4 members were listed, i.e., galectin-1 to -4. In addition, papers describing 3 putative members (galectin-5 to -7) were in preparation [1][2]. Currently, the family comprises 15 members which has allowed further classification into 3 different subtypes based on specific structural features, i.e., prototype (or dimeric), tandem-repeat, and chimera-type galectins (Figure 1a). The prototype galectins (galectin-1/-2/-5/-7/-10/-11/-13/-14/-15) consist of a single CRD which assembles into homo-dimers. Tandem-repeat galectins (galectin-4/-6/-8/-9/-12) have two distinct CRDs that are covalently linked by a short peptide sequence, while the only known chimera-type galectin (galectin-3) consists of a single CRD with an N-terminal non-lectin domain that contributes to protein oligomerization [3][4].
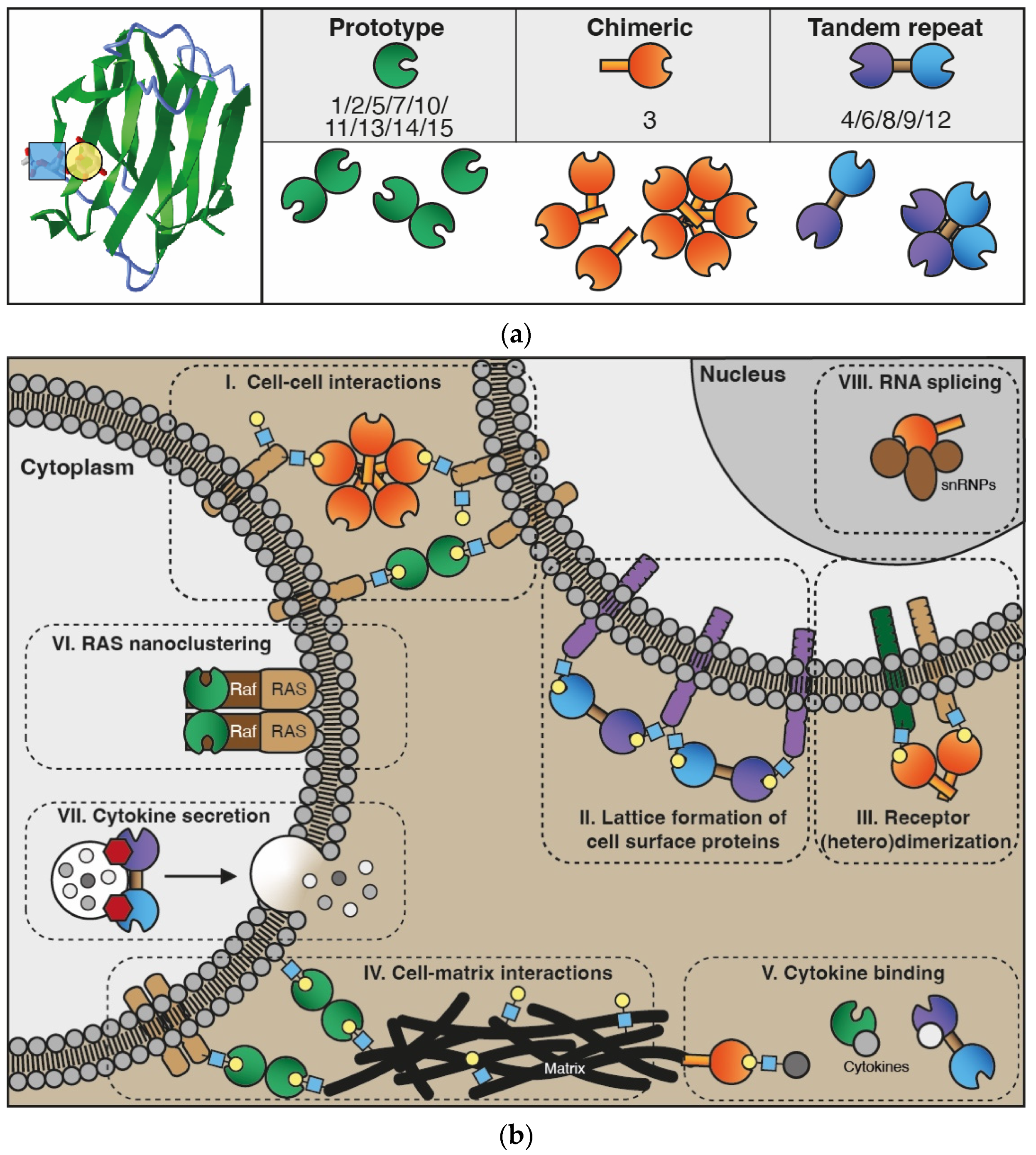
Figure 1. The galectin protein family. (a) Left panel: Cartoon of the anti-parallel beta-sheet structure forming the carbohydrate recognition domain of galectin-1 (in green). On the left, the interaction of a LacNAc (N-acetyllactosamine; blue-yellow) moiety in the binding groove is shown. Right panel: Overview of the 15 galectins that are expressed in humans. See text for explanation of the subgroups. (b) Cartoon depicting some of the key functions of galectins intra- and extracellularly. In the extracellular environment and on the cell surface, galectins can interact with glycoconjugates (blue-yellow) to facilitate, e.g., cell–cell interactions (I) and cell–extracellular matrix interactions (IV) to enable cell adhesion and migration. In addition, extracellular galectins can mediate interactions between molecules in the cell membrane to stimulate, e.g., lattice formation (II) and receptor dimerization (III), thereby promoting receptor cell surface retention and cell signaling. More recently, galectins were also found to heterodimerize with cytokines (V), affecting both galectin and cytokine activity. In the cytoplasm, galectins have been shown to engage in protein–protein interactions that facilitate, e.g., H-Ras nanocluster formation (VI) and signaling as well as cytokine secretion (VII). Finally, in the nucleus, galectins can interact with small nuclear ribonucleoproteins (VIII) thereby contributing to mRNA splicing.
2. Galectins in Esophageal Cancer
2.1. Galectin-1
The first evidence of galectin expression in esophageal cancer was provided by Kayser and coworkers in 2001 [5]. The authors used an immunohistochemical approach to determine the expression of both galectin-1 and galectin-3 in 43 cancer tissues from ESCC patients. In addition, they used labeled galectins to explore the presence of galectin-binding glycans in the tumor tissue [5]. Galectin-1 expression was detected in 25/43 patients (±60%) while galectin-3 expression was detected in 33/43 patients (±80%). Galectin-1 and galectin-3 binding was observed in 35/43 and 27/43 cases, respectively. While galectin binding was not associated with tumor stage or nodal involvement, galectin-1 expression was less frequently observed in lymph node positive vs. lymph node negative patients (33% vs. 71%, p < 0.05). In contrast, Li et al. did not find a significant correlation of galectin-1 positivity with nodal stage after immunohistochemical analysis of expression in tumor tissues obtained from 93 ESCC patients [6]. Roughly half of the patients showed moderate to high expression and the other half showed no or low expression [6]. None of the evaluated clinicopathological parameters, including stage, grade or tumor location, was significantly associated with low (sum of absent/low score) or high (sum of moderate/high score) galectin-1 expression levels. At the same time, survival analysis showed that high galectin-1 expression was significantly correlated with reduced overall survival and disease-free survival. Multivariate analyses confirmed high galectin-1 expression as an independent prognostic factor for overall survival (HR = 2.1, p = 0.001) as well as for disease-free survival (HR = 1.8, p = 0.01) [6]. Thus, it appears that galectin-1 expression might have prognostic value in ESCC patients but this should be confirmed in larger patient groups.
Hoshino and coworkers explored whether the levels of circulating autoantibodies against galectin-1 could be a prognostic biomarker in ESCC [7]. For this, blood serum of 85 ESCC patients was analyzed. In addition, TCGA expression data from 184 ESCC patients were included. Galectin-1 autoantibodies were detected in approximately 10% of patients which was confirmed in a follow up study including more esophageal cancer cases as well as other tumor types [7][8]. While circulating autoantibodies and galectin-1 gene expression was significantly higher in patients vs. healthy controls, there was no significant difference observed with regard to prognosis [7]. Thus, while elevated expression in ESCC tumor tissues might underlie an increase in galectin-1 autoantibodies, the presence of these antibodies did not appear to be a valuable prognostic biomarker. Of note, when galectin-1 was included in a panel with three other autoantibodies, the diagnostic value was comparable to a combination of the classical tumor markers CEA (carcinoembryonic antigen) and SCC-Ag (squamous cell carcinoma antigen), i.e., 32% and 40% sensitivity, respectively [7]. Whether circulating levels of galectin-1 protein itself rather than autoantibodies have diagnostic or prognostic value in esophageal cancer still needs to be established. In that regard, in other tumor types, like colorectal cancer and glioma, it has been found that galectin-1 serum levels can have diagnostic and predictive value [9][10][11]. Moreover, researchers recently described that increased galectin-1 serum levels might serve as a negative predictive biomarker for the response to regorafenib and paclitaxel in patients with advanced esophagogastric cancer (EGC) that were refractory to first-line chemoradiotherapy [12]. Thus, it would be worthwhile to further explore the value of serum galectin-1 as a diagnostic, prognostic or even predictive biomarker in both ESCC and EAC.
Regarding the mechanism(s) responsible for elevated galectin-1 expression, a link has been made with Pituitary Tumor-Transforming Gene 1 (PTTG1) expression. Previously, it was found that PTTG1 is overexpressed in >60% of ESCC patients [13][14]. In addition, increased PTTG1 expression has been correlated to a higher metastatic potential of ESCC cell as it enhanced cell motility and increased the number of lymph node metastases in a mouse model [14]. Of note, high PTTG1 expression was not significantly associated with shorter overall survival [15]. Interestingly, Yan et al. found that increased PTTG1 expression actually induced the expression and secretion of galectin-1 via c-Myc. In addition, the elevated galectin-1 expression was involved in the increased motility and metastatic potential of ESCC cells [13].
More recently, it was described that a long non-coding RNA, i.e., ESCCAL-1 (ESCC-associated lncRNA), interacts with galectin-1 [16]. The interaction hampers ubiquitination of galectin-1 by SMURF1, an E3 ubiquitin-protein ligase. Consequently, elevated ESCCAL-1 expression in ESCC cell lines and patients was associated with higher galectin-1 protein levels. This resulted in enhanced cell proliferation through galectin-1-mediated cell cycle progression [16]. Thus, the increased levels of galectin-1 in ESCC could in part be related to induction of expression as well as to reduced protein turnover. Since galectin-1 expression is also known to be induced by cytokines [17][18] and under pro-angiogenic conditions [19] it is worthwhile to further explore which factors contribute to the observed high protein levels in ESCC patients.
Overall, current evidence suggests that galectin-1 is involved in ESCC progression which is in line with other tumor types. This warrants further investigation into the exact regulation and function of galectin-1, not only in ESCC but also in EAC.
2.2. Galectin-3
As mentioned above, Kayser and colleagues were also the first to report on galectin-3 expression in ESCC. However, in contrast to galectin-1, the authors did not find any association between galectin-3 positivity and nodal involvement [5]. Another group also used immunohistochemical staining to evaluate whether galectin-3 was a prognostic marker for esophageal cancer patients. The study comprised 63 patients (62 ESCC and 1 EAC) with locally advanced esophageal cancer that received neoadjuvant chemoradiotherapy [20]. High expression was observed in 18 cases (29%) but no association with clinicopathological parameters or survival outcome was observed [20]. Similar observations were made in a study that included 154 ESCC tissues from patients that were treated by surgical resection without neoadjuvant treatment [21]. In this study, the authors also made a distinction between nuclear and cytoplasmic galectin-3 staining. In line with the previous findings, high cytoplasmic galectin-3 levels (≥45% positive cells, 72 cases) showed no significant association with clinicopathological parameters [21]. Furthermore, while high nuclear levels (≥30% positive cells, 23 cases) inversely correlated with vascular invasion and histological differentiation, neither cytoplasmic nor nuclear galectin-3 was prognostic [21]. Thus, the role of galectin-3 in ESCC appears to be minor.
Despite the limited diagnostic and prognostic value of galectin-3 in esophageal cancer, Balasubramanian and colleagues included 52 esophageal carcinoma patients in a study that evaluated whether galectin-3 levels in urine could be used to monitor disease status and/or treatment efficacy [22]. Compared to healthy controls, urine galectin-3 levels were significantly higher in esophageal cancer patients, most notably in patients with metastatic disease. In fact, galectin-3 levels significantly correlated with disease stage which is different from the observations reported in esophageal tumor tissues [22]. However, the study using urine samples did not specify the types of esophageal cancer so these opposing findings might reflect a difference between ESCC and EAC which needs further investigation.
Regarding the functional role of galectin-3 expression in esophageal cancer, Liang and colleagues studied the effect of increased galectin-3 expression on ESCC cell behavior. For this, the authors virally transduced an ESCC cell line (Eca-109) in order to overexpress galectin-3 [23]. Subsequently, different functional features of parental and galectin-3 overexpressing cells were compared, including proliferation, apoptosis, migration and invasion. The parental cells already showed considerable galectin-3 protein expression and in the transduced cells this level increased only ±1.5 fold. Nevertheless, the transduced cells displayed significantly lower apoptosis and an increased proliferative, migratory and invasive capacity [23]. Thus, despite the limited diagnostic or prognostic value, galectin-3 might be involved in regulation of ESCC progression and present as a target for treatment. The latter was explored by the same group in a follow-up study. Using the same cell line and an siRNA approach, the authors were able to significantly inhibit galectin-3 protein levels by 65 to 90% [24]. An inhibitory effect of knockdown on proliferation became apparent 72 h after siRNA transfection. In addition, migration and invasion were hampered while apoptosis levels increased following galectin-3 knockdown [24]. Both studies suggest that galectin-3 expression could serve as a potential therapeutic target in ESCC. In line with this, Cui et al. investigated the effect of targeting galectin-3 in the context of resistance to EGFR-targeted therapy with gefitinib [25]. The authors found that siRNA-mediated knockdown of galectin-3 sensitized the ESCC cell line TE-8 to gefitinib treatment. This was associated with reduced internalization of EGFR in the knockdown cells. This was confirmed in an in vivo tumor model, in which galectin-3 knockdown sensitized tumors to gefitinib to a similar extend as inhibition of dynamin-dependent endocytosis [25]. Finally, Xu and colleagues found that synephrine, a compound isolated from citrus tree leaves, effectively inhibited the proliferation, migration, invasion and colony formation of 2 ESCC cell lines (KYSE30 + KYSE270) [26]. The compound also hampered in vivo tumor growth in a xenograft mouse model and sensitized cells to 5-FU treatment. Importantly, subsequent proteomic analyses indicated reduced AKT and ERK signaling as key effects with galectin-3 as the upstream regulatory protein [26]. In line with this, synephrine treatment reduced galectin-3 expression in the ESCC cells [26]. Together with the findings by Cui et al., this suggests that targeting galectin-3 could be beneficial for ESCC patients as it has direct effects on the tumor cells and sensitizes the cells for combination treatment.
Collectively, despite the limited diagnostic and prognostic value of galectin-3 in ESCC, the protein might serve as a therapeutic target, in particular in combination with other therapeutics in this subtype. However, in line with galectin-1, hardly anything is known regarding galectin-3 in EAC and more research is required to establish the therapeutic value and function in both subtypes.
2.3. Galectin-7
A possible role of galectin-7 in esophageal cancer has been reported by only 1 study. Zhu and coworkers observed higher galectin-7 protein levels in ESCC tissue after proteomic analyses of patient-matched normal and tumor samples [27]. The elevated expression was confirmed in independent sets of patient samples which showed high expression in more than 50% of all cases. Overall, an approximately 2.5-fold increase was found in tumor vs. normal tissue. In addition, it was observed that galectin-7 protein was detectable in the nucleus, cytoplasm and on the membrane of cells [27]. Interestingly, within the ESCC samples, well differentiated (grade I) tumors expressed significantly higher levels of galectin-7 as compared to poorly differentiated (grade III) tumors. Of note, using a comparable proteomic approach, Qi et al. did not identify increased galectin-7 expression in ESCC [28]. This might be related to experimental setup and/or specific protein spot selection after 2D gel electrophoresis but it also indicates that deciphering the role of galectin-7 in ESCC requires further studies.
2.4. Galectin-9
Regarding the role of galectin-9 in esophageal cancer, Hou and coworkers analyzed the expression of galectin-9 and its binding partner Tim-3 in tumor tissues obtained from 45 ESCC patients during curative surgical resection. None of the patients received neo-adjuvant treatment [29]. Based on immunohistochemical staining, 7 patients were classified as ‘galectin-9 high’ while 38 were classified as ‘galectin-9 low’. Of the latter, 11 cases did not show any galectin-9 staining at all. While the galectin-9 expression was not significantly associated with any clinicopathological parameters, survival analyses showed that patients in the galectin-9 high group had significantly longer disease-free survival as compared to patients in the galectin-9 low group. However, subsequent multivariate Cox regression analysis did not identify galectin-9 as an independent prognostic factor in this patient cohort [29]. Since the number of patients included in this study was limited, additional research is required to determine whether galectin-9 has any prognostic value in ESCC. Of note, there was a negative correlation between galectin-9 expression and Tim-3 expression [29].
The effect of exogenously added galectin-9 on different esophageal cancer cell lines has also been explored. In EAC cell lines, galectin-9 treatment dose-dependently inhibited tumor cell proliferation and at the same time induced caspase-independent apoptosis [30]. Further research focused on a single cell line and indicated that galectin-9 might inhibit the autophagy flux, while the effects on cell cycle regulatory proteins and cell cycle progression were minimal and absent, respectively [30]. Apparently, galectin-9 inhibits EAC cell growth mainly via induction of apoptosis. Of note, the authors observed that galectin-9 also induced CXCL8 (IL-8) secretion by the cancer cells. In ESCC, increased CXCL8 expression was previously shown to be a predictive marker for poor prognosis [31], so it is tempting to speculate that this is also related to galectin-9 expression. At the same time, since galectin-9 hampers tumor growth it is difficult to reconcile galectin-9 induction of CXCL8 with poor prognosis. Nevertheless, in a follow-up study by the same group, the growth inhibitory and pro-apoptotic effects of galectin-9 were confirmed in esophageal squamous cell carcinomas using different in vitro and in vivo models [32]. Moreover, it was suggested that apoptosis was induced via activation of the intrinsic pathway, i.e., mitochondria-mediated apoptosis [32]. Unfortunately, CXCL8 expression or secretion was not assessed.
The data on galectin-9 are limited but suggest that this family member might serve as a prognostic marker and therapeutic protein in esophageal cancer. In EAC, the protein hampers tumor progression which is in line with the observation in ESCC that high galectin-9 expression is associated with better overall survival. These findings warrant further studies of this galectins in esophageal cancer.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14235790
References
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.; et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598.
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994, 269, 20807–20810.
- Rabinovich, G.A.; Toscano, M.A. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352.
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884.
- Kayser, K.; Hauck, E.; André, S.; Bovin, N.V.; Kaltner, H.; Banach, L.; Lancaster, E.; Gabius, H.J. Expression of endogenous lectins (galectins, receptors for ABH-epitopes) and the MIB-1 antigen in esophageal carcinomas and their syntactic structure analysis in relation to post-surgical tumor stage and lymph node involvement. Anticancer. Res. 2001, 21, 1439–1444.
- Li, S.H.; Chen, Y.H.; Lu, H.I.; Lo, C.M.; Huang, C.C.; Wang, Y.M.; Huang, E.Y. Galectin-1 Expression Is Associated with the Response and Survival Following Preoperative Chemoradiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma. Cancers 2021, 13, 3147.
- Hoshino, I.; Nabeya, Y.; Takiguchi, N.; Gunji, H.; Ishige, F.; Iwatate, Y.; Kuwajima, A.; Shiratori, F.; Okada, R.; Shimada, H. Inducing multiple antibodies to treat squamous cell esophageal carcinoma. BMC Cancer 2020, 20, 1007.
- Nanami, T.; Hoshino, I.; Shiratori, F.; Yajima, S.; Oshima, Y.; Suzuki, T.; Ito, M.; Hiwasa, T.; Kuwajima, A.; Shimada, H. Prevalence of serum galectin-1 autoantibodies in seven types of cancer: A potential biomarker. Mol. Clin. Oncol. 2021, 15, 179.
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta 2015, 1855, 235–247.
- Watanabe, M.; Takemasa, I.; Kaneko, N.; Yokoyama, Y.; Matsuo, E.; Iwasa, S.; Mori, M.; Matsuura, N.; Monden, M.; Nishimura, O. Clinical significance of circulating galectins as colorectal cancer markers. Oncol. Rep. 2011, 25, 1217–1226.
- Verschuere, T.; Van Woensel, M.; Fieuws, S.; Lefranc, F.; Mathieu, V.; Kiss, R.; Van Gool, S.W.; De Vleeschouwer, S. Altered galectin-1 serum levels in patients diagnosed with high-grade glioma. J. Neurooncol. 2013, 115, 9–17.
- Stroes, C.I.; Schokker, S.; Khurshed, M.; van der Woude, S.O.; Mathôt, R.A.; Slingerland, M.; de Vos-Geelen, J.; Zucchetti, M.; Matteo, C.; van Dijk, E.; et al. A phase Ib/II study of regorafenib and paclitaxel in patients with beyond first-line advanced esophagogastric carcinoma (REPEAT). Adv. Med. Oncol. 2022, 14, 17588359221109196.
- Yan, S.; Zhou, C.; Lou, X.; Xiao, Z.; Zhu, H.; Wang, Q.; Wang, Y.; Lu, N.; He, S.; Zhan, Q.; et al. PTTG overexpression promotes lymph node metastasis in human esophageal squamous cell carcinoma. Cancer Res. 2009, 69, 3283–3290.
- Ito, T.; Shimada, Y.; Kan, T.; David, S.; Cheng, Y.; Mori, Y.; Agarwal, R.; Paun, B.; Jin, Z.; Olaru, A.; et al. Pituitary tumor-transforming 1 increases cell motility and promotes lymph node metastasis in esophageal squamous cell carcinoma. Cancer Res. 2008, 68, 3214–3224.
- Chen, F.F.; Zhang, S.R.; Peng, H.; Chen, Y.Z.; Cui, X.B. Integrative genomics analysis of hub genes and their relationship with prognosis and signaling pathways in esophageal squamous cell carcinoma. Mol. Med. Rep. 2019, 20, 3649–3660.
- Cui, Y.; Yan, M.; Wu, W.; Lv, P.; Wang, J.; Huo, Y.; Lou, Y.; Ma, X.; Chang, J.; Guan, F.; et al. ESCCAL-1 promotes cell-cycle progression by interacting with and stabilizing galectin-1 in esophageal squamous cell carcinoma. NPJ Precis. Oncol. 2022, 6, 12.
- Thijssen, V.L.; Rabinovich, G.A.; Griffioen, A.W. Vascular galectins: Regulators of tumor progression and targets for cancer therapy. Cytokine Growth Factor Rev. 2013, 24, 547–558.
- He, J.; Baum, L.G. Endothelial cell expression of galectin-1 induced by prostate cancer cells inhibits T-cell transendothelial migration. Lab. Investig. 2006, 86, 578–590.
- Thijssen, V.L.; Postel, R.; Brandwijk, R.J.; Dings, R.P.; Nesmelova, I.; Satijn, S.; Verhofstad, N.; Nakabeppu, Y.; Baum, L.G.; Bakkers, J.; et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. USA 2006, 103, 15975–15980.
- Kang, S.Y.; Han, J.H.; Lee, K.J.; Choi, J.-H.; Park, J.I.; Kim, H.I.; Lee, H.-W.; Jang, J.H.; Park, J.S.; Kim, H.C.; et al. Low expression of Bax predicts poor prognosis in patients with locally advanced esophageal cancer treated with definitive chemoradiotherapy. Clin. Cancer Res. 2007, 13, 4146–4153.
- Shibata, T.; Noguchi, T.; Takeno, S.; Takahashi, Y.; Fumoto, S.; Kawahara, K. Impact of nuclear galectin-3 expression on histological differentiation and vascular invasion in patients with esophageal squamous cell carcinoma. Oncol. Rep. 2005, 13, 235–239.
- Balasubramanian, K.; Vasudevamurthy, R.; Venkateshaiah, S.U.; Thomas, A.; Vishweshwara, A.; Dharmesh, S.M. Galectin-3 in urine of cancer patients: Stage and tissue specificity. J. Cancer Res. Clin. Oncol. 2009, 135, 355–363.
- Liang, N.; Song, X.; Xie, J.; Xu, D.; Liu, F.; Yu, X.; Tian, Y.; Liu, Z.; Qiao, L.; Zhang, J. Effect of galectin-3 on the behavior of Eca-109 human esophageal cancer cells. Mol. Med. Rep. 2015, 11, 896–902.
- Qiao, L.; Liang, N.; Xie, J.; Luo, H.; Zhang, J.; Deng, G.; Li, Y.; Zhang, J. Gene silencing of galectin-3 changes the biological behavior of Eca109 human esophageal cancer cells. Mol. Med. Rep. 2016, 13, 160–166.
- Cui, G.; Cui, M.; Li, Y.; Liang, Y.; Li, W.; Guo, H.; Zhao, S. Galectin-3 knockdown increases gefitinib sensitivity to the inhibition of EGFR endocytosis in gefitinib-insensitive esophageal squamous cancer cells. Med. Oncol. 2015, 32, 124.
- Xu, W.W.; Zheng, C.C.; Huang, Y.N.; Chen, W.Y.; Yang, Q.S.; Ren, J.Y.; Wang, Y.M.; He, Q.Y.; Liao, H.X.; Li, B. Synephrine Hydrochloride Suppresses Esophageal Cancer Tumor Growth and Metastatic Potential through Inhibition of Galectin-3-AKT/ERK Signaling. J. Agric. Food Chem. 2018, 66, 9248–9258.
- Zhu, X.; Ding, M.; Yu, M.-L.; Feng, M.-X.; Tan, L.-J.; Zhao, F.-K. Identification of galectin-7 as a potential biomarker for esophageal squamous cell carcinoma by proteomic analysis. BMC Cancer 2010, 10, 290.
- Qi, Y.; Chiu, J.F.; Wang, L.; Kwong, D.L.; He, Q.Y. Comparative proteomic analysis of esophageal squamous cell carcinoma. Proteomics 2005, 5, 2960–2971.
- Hou, N.; Ma, J.; Li, W.; Zhao, L.; Gao, Q.; Mai, L. T-cell immunoglobulin and mucin domain-containing protein-3 and galectin-9 protein expression: Potential prognostic significance in esophageal squamous cell carcinoma for Chinese patients. Oncol. Lett. 2017, 14, 8007–8013.
- Akashi, E.; Fujihara, S.; Morishita, A.; Tadokoro, T.; Chiyo, T.; Fujikawa, K.; Kobara, H.; Mori, H.; Iwama, H.; Okano, K.; et al. Effects of galectin-9 on apoptosis, cell cycle and autophagy in human esophageal adenocarcinoma cells. Oncol. Rep. 2017, 38, 506–514.
- Ogura, M.; Takeuchi, H.; Kawakubo, H.; Nishi, T.; Fukuda, K.; Nakamura, R.; Takahashi, T.; Wada, N.; Saikawa, Y.; Omori, T.; et al. Clinical significance of CXCL-8/CXCR-2 network in esophageal squamous cell carcinoma. Surgery 2013, 154, 512–520.
- Chiyo, T.; Fujita, K.; Iwama, H.; Fujihara, S.; Tadokoro, T.; Ohura, K.; Matsui, T.; Goda, Y.; Kobayashi, N.; Nishiyama, N.; et al. Galectin-9 Induces Mitochondria-Mediated Apoptosis of Esophageal Cancer In Vitro and In Vivo in a Xenograft Mouse Model. Int. J. Mol. Sci. 2019, 20, 2634.
This entry is offline, you can click here to edit this entry!
