Biodiesel constitutes an attractive source of energy because it is renewable, biodegradable, and non-polluting. Up to 20% biodiesel can be blended with fossil diesel and is being produced and used in many countries. Biodiesel is produced through the transesterification reaction of fat waste with a short-chain alcohol, usually methanol, in the presence of a catalyst. Animal fats, usually found as waste from slaughterhouses, meat processing industry, and cooking facilities, constitute an important waste with costly treatment that can be reduced if used as feedstock for biodiesel production. Animal fat waste represents near 6% of total feedstock used to produce biodiesel through alkaline catalysis transesterification after its pretreatment. Lipase transesterification has some advantages such as the requirement of mild conditions, absence of pretreatment, no soap formation, simple downstream purification process and generation of high quality biodiesel. However, it has some disadvantages like the cost of the enzyme, its poor stability, and the enzyme deactivation by alcohol, that can be partly overcome through enzyme immobilization. A few companies are using liquid lipase formulations and, in some cases, immobilized lipases for industrial biodiesel production.
Biodiesel constitutes an attractive source of energy because it is renewable, biodegradable, and non-polluting. Up to 20% biodiesel can be blended with fossil diesel and is being produced and used in many countries. Biodiesel is produced through the transesterification reaction of fat waste with a short-chain alcohol, usually methanol, in the presence of a catalyst. Animal fats, usually found as waste from slaughterhouses, meat processing industry, and cooking facilities, constitute an important waste with costly treatment that can be reduced if used as feedstock for biodiesel production. Animal fat waste represents near 6% of total feedstock used to produce biodiesel through alkaline catalysis transesterification after its pretreatment. Lipase transesterification has some advantages such as the requirement of mild conditions, absence of pretreatment, no soap formation, simple downstream purification process and generation of high quality biodiesel. However, it has some disadvantages like the cost of the enzyme, its poor stability, and the enzyme deactivation by alcohol, that can be partly overcome through enzyme immobilization. A few companies are using liquid lipase formulations and, in some cases, immobilized lipases for industrial biodiesel production.
- biodiesel
- fuel
- energy generation
- food waste
- animal waste
- animal fat
- enzymatic transesterification
1. Introduction
Animal byproducts are subjected to rendering where fat such as beef tallow, mutton tallow, pork lard, and chicken fat are obtained [1][2]. Such fat is majorly composed of triacylglycerols with fatty acids of 16 to 18 carbons. The most abundant saturated fatty acids are palmitic (16:0) and stearic (18:0) acids; the major monounsaturated fatty acid is oleic acid (18:1) and the most abundant polyunsaturated fatty acids are linoleic (18:2) and arachidonic (20:4) acids [3][4]. Animal fat waste is also obtained from the meat processing industry and from recycled waste from the cooking business [5][6] and are classified as yellow grease if the content of free fatty acids is lower than 15% by weight and brown grease when it is higher than 15% [7]. In 2019, more than 800 thousand tons of animal fats, equivalent to 6% of total feedstock, were used to produce biodiesel in the European Union [8][9], while 8.4% of total feedstock was used in the case of the US, consisting of mainly 74 tons of poultry fat, 132 tons of tallow and 243 thousand tons of white grease [10].
Biodiesel produced from animal fats has several advantages in comparison to that produced from vegetable oils. So, it has lower production cost because the feedstock used as raw material for biodiesel production represents up to 80% of the total cost [11], and the fossil CO2 reduction is higher (nearly 80% CO2 reduction may be reached for animal fat in comparison to 30% reduction when using vegetable oil) [12][13][14]. The bioenergy demand is continuously increasing and in 2050 it is expected to reach 30% of the fuel consumed in the world for road transport [14][15]. Research on biodiesel production is trying to maximize the yield and minimize the costs by using better catalysts that can be reused and improve the transesterification efficiency [16][17].
Total biodiesel world production has been increasing progressively year by year, reaching nearly 45 million tons in 2019 [9]. The European Union has the largest biodiesel production through its 202 plants producing more than 14 million tons of biodiesel in 2019 [8][18]. More than 5.6 million tons of biodiesel were produced in the US in 2019 through its 91 plants [10][19]. Nearly 80% of new diesel vehicles are prepared for B20 use that consists of fossil diesel blended with 20% biodiesel [10].
Transesterification through alkaline catalysis is the preferred process at industrial biodiesel production plants [20]. However, raw materials like animal fat that contain moisture and free fatty acids are troublesome for alkaline transesterification due to soap formation. Acid catalysis does not have such troubles, but the reaction is much slower than alkaline catalysis, it needs a larger size reactor and requires a higher alcohol to fat molar ratio [21]. Heterogeneous catalysts are not sensitive to the presence of free fatty acids and moisture, can catalyze esterification and transesterification simultaneously, and can be separated from the reaction media. However, such solid catalysts tend to form three phases resulting in a reduced reaction rate and high energy consumption [22]. The simultaneous esterification and transesterification also occur with supercritical technology where high temperature and pressure conditions (i.e., >250 °C and 10 MPa) increase the solubility and reduce the mass transfer limitation resulting in good efficiency but with high energy consumption [23][24][25]. Pseudo catalytic transesterification using biochar as the porous material for the pseudo-catalytic reaction at more than 300 °C has the same advantages as supercritical transesterification, but also has high energy consumption [26][27]. Therefore, an interesting alternative for biodiesel production from animal fats is the enzymatically catalyzed transesterification that is presented in this article.
2. Mechanisms of Action of Lipases
Lipases, triacylglycerol ester hydrolases (EC 3.1.1.3), are serine hydrolases with an active site containing an amino-acid triad of serine, histidine and aspartate [28]. Lipases are obtained from a variety of sources such as animal and plant tissues and microorganisms. Lipases show a wide range of pH and temperature for activity and vary from strain to strain regarding specificity and hydrolysis rate [29] Lipases exhibit good stability in non-aqueous mediums and exhibit maximum activity near neutral pH range; lipase stability is increased when the enzyme is immobilized.
Lipases can catalyze esterification, inter-esterification, and trans-esterification reactions in non-aqueous environments. Lipases catalyze the hydrolysis of triacylglycerols at the aqueous-non aqueous interface but these enzymes can also catalyze the synthesis of esters from alcohols and long chain fatty acids in low moisture environment [29]. Lipases follow a two-step mechanism for the generation of fatty acid methyl esters in transesterification reactions, usually through the Ping-Pong Bi Bi mechanism [30].
Most triacylglycerol lipases are regiospecific because they can only hydrolyze primary ester bonds at the sn-1 and sn-3 positions, external positions within the triacylglycerol, and can generate either one free fatty acid and diacylglycerol, or two free fatty acids and 2-monoacylglycerol that remain unhydrolyzed. The full process from triacylglycerols into biodiesel and glycerol as end products is shown in Figure 1. Regiospecificity is characteristic of extracellular bacterial lipases from Bacillus sp. [31][32].
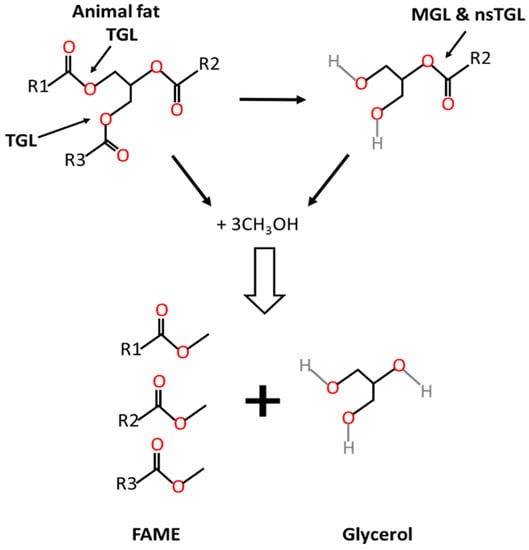
Figure 1. Transesterification of animal fat to biodiesel. TGL: triacylglycerol lipase; nsTGL: non specific triacylglycerol lipase; MGL: monoacylglycerol lipase.
Monoacylglycerol lipases (EC 3.1.1.23) catalyze the hydrolysis at the specific sn-2 position of 2-monoacylglycerol into free fatty acid and glycerol. Such lipases may be present in the enzyme extract and masked when measuring activity with standard activity methods like those based on triolein hydrolysis measurement. Monoacylglycerol lipases have been the object of few studies [33], although they might be present in some microbial enzyme preparations [34]. Other lipases are nonspecific and can act on any of the ester bonds of the triacylglycerol and therefore break down the triacylglycerol to release free fatty acids and glycerol as the final products. This is the case of lipases from Staphylococcus aureus [35], Geotrichum candidum, Corynebacterium acnes, Penicillium cyclopium [21] and Chromobacterium viscosu [36]. Another alternative for the hydrolysis of monoacylglycerols is the acyl migration in the glycerol backbone from the sn-2 position to sn-1 or sn-3 positions [37].
The specificity of lipases depends on the length of fatty acids, presence of double bonds, branched groups and, consequently, reaction rates may have important variations depending on the composition of triacylglycerols present in the fat waste. Lipases are especially active against medium to long chain fatty acids, which are those more usual in animal fat waste [16].
3. Sources of Lipases
Most lipases originated from microorganisms are produced in fermenters under controlled conditions (see Table 1). Lipases are produced by a variety of gram-positive and gram-negative bacterial strains, especially from the genera of Pseudomonas [38][39], also by filamentous fungus that are commercially important such as those belonging to the genera of Rhizopus sp. [40], Aspergillus sp. [41], Penicillium sp. [42], Geotrichum sp. [29], Mucor sp. [43] and Thermomyces sp. [44]. Lipases produced from yeasts are also relevant such as those from Candida sp. [45,46].
| Lipase Origin | Reference |
|---|---|
| Pseudomonas fluorescens | [47] |
| Burkholderia cepacia | [38,46.48,49] |
| Staphylococcus haemolyticus | [50] |
| Chromobacterium viscosum | [51] |
| Phichia pastoris | [52] |
| Mucor miehei | [53] |
| Thermomyces lanuginosus | [54,55] |
| Aspergillus oryzae | [56] |
| Aspergillus niger | [57,58] |
| Aspergillus terreus | [59] |
| Rhizopus oryzae | [37,60,61] |
| Rhizomucor miehei | [55,62] |
| Geotrichum candidum | [63] |
| Candida antarctica | [61,64,65,66] |
| Candida cylindracea | [67] |
| Candida rugosa | [46,68,69] |
Extracellular lipases are secreted into the production medium and recovered from the microorganism broth. Then, lipases are further separated and purified but downstream processing is costly. Intracellular lipases imply the use of whole cell microorganisms and this fact reduces the costs of enzyme extraction and purification but the efficiency and biodiesel yield is low when catalyzing an oily substrate due to mass transfer limitations for substrate penetration and product release [25,70]. Some whole cell biocatalysts used to produce biodiesel are filamentous fungi like Aspergillus and Rhizopus [45].
4. Industrial Applications of Lipase-Catalyzed Biodiesel
Even though transesterification through alkaline catalysis is the preferred process in the majority of industrial biodiesel production plants [20], a few lipase-based processes have already been implemented to plant-scale operation. Lipases generate biodiesel under mild reaction conditions through the conversion of free fatty acids and triacylglycerols in the presence of an acyl acceptor [28]. However, it has some disadvantages like the cost of the enzyme, its poor stability, and the enzyme deactivation by alcohol [71], and partly by the generated glycerol [61]. Most of these issues can be partly overcome through enzyme immobilization [72,73] because it increases its stability and efficiency [74,75,76], and also allows an easy downstream separation from the product and decreases costs [77]. The immobilization of lipases consists of the retention of the enzyme at the surface of the support material. In this way, immobilized lipases show an improved efficiency and reduced costs, with longer enzyme stability and better resistance to denaturation by alcohol. There are many available supports of organic, synthetic, and inorganic nature for lipase immobilization and there is a large variety of immobilization procedures such as adsorption, covalent binding, cross-linking, entrapment, or encapsulation [73].
There are some industrial applications of enzyme transesterification for biodiesel production in different countries. The collaboration of Novozymes (Bagsvaerd, Denmark) with Piedmont Biofuels (Pittsboro, NC, USA) resulted in a patent application to produce fatty acid alkyl esters, by a lipolytic enzyme in a solution containing triacylglycerol, alcohol, water, and glycerol [78,79]. Viesel Fuel (Terrac Stuart, FL, USA) upgraded in 2013 its facility through an enzymatic process developed by Novozymes (Denmark) to use brown grease and waste cooking oil to produce up to 11 million gallons biodiesel per year using Eversa Transform® lipase from Novozymes, a soluble lipase produced by a genetically modified strain of Aspergillus oryzae [80], and an ion exchange resin system for removal of remaining free fatty acids during crude biodiesel refining [81,82]. Viesel Fuel, Novozymes and Tactical Fabrication also collaborated with Buster Biofuels to upgrade its facility in San Diego (CA, USA) to produce up to 5 million gallons per year [83]. Lvming and Environmental Protection Technology Co. Ltd. (Shanghai, China) used lipase of Candida sp. to produce 10,000 tons per year from waste frying oil [84]. A plant in Sumaré (Sao Paulo, Brazil) produces biodiesel from mixed beef tallow and soybean oil using Callera® Trans L lipase in a batch reactor [85]. These companies are using liquid lipase formulations but the efficiency of the process can be improved further by using recent developments in immobilized lipases. So, Hunan Rivers Bioengineering Co. Ltd. (Hunan, China) was reported to use Novozym 435® lipase in a stirred tank reactor to produce 20,000 tons of biodiesel per year. The enzyme is a lipase B from Candida antarctica immobilized on a resin consisting of macroporous support formed by poly(methyl methacrylate) crosslinked with divinylbenzene [86]. New technology protected with patents [87] has been provided by EnzymoCore, a leading global producer company founded in 2007 in Israel and with several active biodiesel plants around the world. This company has developed modified-immobilized enzymes, supported on solid organic resins, with high resistance to methanol and able to produce biodiesel from any type of oil or fat, even those cheap and with very large content of free fatty acids and polar lipids [88].
5. Conclusions
Animal fat waste, usually resulting from slaughterhouses, the meat processing industry, and cooking facilities, is being increasingly used as feedstock for biodiesel production. Transesterification through alkaline catalysis is the preferred process at industrial biodiesel production plants although some enzymatic transesterification processes have been already implemented to plant-scale operation. Transesterification with lipases has traditional problems including poor enzyme stability, difficulties in reusability, and denaturation by alcohol although they can be partly overcome through enzyme immobilization. An advantage is that lipases are not affected by water and free fatty acids typically found in animal fats. However, some companies have been able to solve such troubles since they are running liquid lipase formulations for producing biodiesel from cooking oil waste at industrial scale although the efficiency of the process can be further improved. Recent developments in immobilized lipases and availability of different types of supports such as mesoporus materials, silica nanoflowers, pickering emulsion, and metal-organic frameworks demonstrate improved efficiency and reduced costs. Immobilization of the enzyme in such materials increases its stability and makes it more resistant to denaturation by alcohol. Magnetic nanomaterials constitute a very good support for enzyme immobilization because they can be recovered when an external magnetic field is applied. These nanoparticles are functionalized on the surface by coating with silica or organic polymers that enhance the efficiency of the process. The entrapment of whole cells with lipase activity, appears to be simple and efficient although more research is needed. Coimmobilization of lipases is an innovative process, but not so attractive for industrial application. It needs further research because of the cost of using different lipases and the steric difficulties for enzymes to hydrolyze triacylglycerols that affects the efficiency of the process.
This entry is adapted from the peer-reviewed paper 10.3390/app10155085
References
- Akhil, U.S.; Alagumalai, A. A Short Review on Valorization of Slaughterhouse Wastes for Biodiesel Production. ChemistrySelect 2019, 4, 13356–13362, 10.1002/slct.201903739
- Barik, D.; Vijayaraghavan, R. Effects of waste chicken fat derived biodiesel on the performance and emission characteristics of a compression ignition engine. Int. J. Ambient Energy 2018, 41, 88–97, 10.1080/01430750.2018.1451370
- Prates, J.; Alfaia, C.; Alves, S.; Bessa, R. Fatty acids. In Handbook of Analysis of Edible Animal by-Products; Nollet, L.M.L., Toldrá, F., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 137–159.
- Mora, L.; Toldrá-Reig, F.; Prates, J.A.M.; Toldrá, F. Cattle by-products. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma and Fuels; Simpson, B.K., Aryee, A.N., Toldrá, F., Eds.; Wiley: Chichester, West Sussex, UK, 2020; pp. 43–55.
- Baladincz, P.; Hancsók, J. Fuel from waste animal fats. Chem. Eng. J. 2015, 282, 152–160, 10.1016/j.cej.2015.04.003
- Mora, L.; Toldrá-Reig, F.; Reig, M.; Toldrá, F. Possible uses of processed slaughter by-products. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press/Elsevier: London, UK, 2019; pp. 145–160.
- Banković-Ilić, I.B.; Stojković, I.J.; Stamenković, O.S.; Veljkovic, V.B.; Hung, Y-T. Waste animal fats as feed stocks for biodiesel production. Sustain. Energy Rev. 2014, 32, 238–254, 10.1016/j.rser.2014.01.038
- Ramos, M.; Dias; Puna; Gomes, J.F.; Bordado, J.; Dias, A.P.S.; Puna, J. Biodiesel Production Processes and Sustainable Raw Materials. Energies 2019, 12, 4408, 10.3390/en12234408
- Flach, B.; Lieberz, S.; Bolla, S. EU Biofuels Annual 2019, Gain Report NL9022, USDA Foreign Agricultural Service. 2019. Available online: http://gain.fas.usda.gov/Pages/Default.aspx Retrieved 2020-5-6
- US Energy Information Administration. Monthly Biodiesel Production Report; US Department of Energy: Washington, DC, USA, 2020. Available online: https://www.eia.gov/biofuels/biodiesel/production/biodiesel.pdf Retrieved 2020-5-5
- Bušić, A.; Kundas, S.; Morzak, G.; Belskaya, H.; Marđetko, N.; Santek, M.I.; Komes, D.; Novak, S.; Šantek, B. Recent Trends in Biodiesel and Biogas Production. Food Technol. Biotechnol. 2018, 56, 152–173, 10.17113/ftb.56.02.18.5547
- IPPR. Time for Change: A New Vision for the British Economy—The Interim Report of the IPPR Commission on Economic Justice. 2017. Available online: http://www.ippr.org/cej-time-for-change Retrieved 2020-3-25
- IRENA. Global Energy Transformation: A Roadmap to 2050. 2018. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2018/Apr/IRENA_Report_GET_2018.pdf Retrieved 2020-4-14
- Rosson, E.; Sgarbossa, P.; Pedrielli, F.; Mozzon, M.; Bertani, R. Bioliquids from raw waste animal fats: An alternative renewable energy source. Biomass Convers. Biorefin. 2020, 1–16, 10.1007/s13399-020-00634-z
- Cernat, A.; Pana, C.; Negurescu, N.; Lazaroiu, G.; Nutu, C.; Fuiorescu, D.; Toma, M.; Nicolici, A. Combustion of preheated raw animal fats-diesel fuel blends at diesel engine. J. Therm. Anal. Calorim. 2019, 140, 2369–2375, 10.1007/s10973-019-08972-5
- Toldrá-Reig, F.; Mora. L.; Toldrá, F. Trends in biodiesel production from animal fat waste. Appl. Sci. 2020, 10, 3644, 10.3390/app10103644
- Lawan, I.; Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. Synergies between the microwave reactor and CaO/zeolite catalyst in waste lard biodiesel production. Renew. Energy 2020, 145, 2550–2560, 10.1016/j.renene.2019.08.008
- Bockey, D. The significance and perspective of biodiesel production—A European and global view. OCL 2019, 26, 40, 10.1051/ocl/2019042.
- Biodiesel. Available online: https://www.biodiesel.org/what-is-biodiesel/biodiesel-basics Retrieved 2020-3-21
- Kristi, M.; Milbrandt, A.; Lewis, J.; Schwab, A. Bioenergy Industry Status 2017 Report; National Renewable Energy Laboratory: Golden, CO, USA, 2018. Available online: https://www.nrel.gov/docs/fy20osti/75776.pdf Retrieved 2020-5-5
- Canakci, M.; Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441, 10.1007/s10295-008-0337-6
- Wancura, J.H.C.; Tres, M.V.; Jahn, S.L.; de Oliveira, J.V. Lipases in liquid formulation for biodiesel production: Current status and challenges. Biotechnol. Appl. Biochem. 2019, 1-20, 10.1002/bab.1835
- Shin, H.-Y.; Lee, S.-H.; Ryu, J.-H.; Bae, S.-Y. Biodiesel production from waste lard using supercritical methanol. J. Supercrit. Fluids 2012, 61, 134–138, 10.1016/j.supflu.2011.09.009
- Marulanda, V.F.; Anitescu, G.; Tavlarides, L.L. Investigations on supercritical transesterification of chicken fat for biodiesel production from low-cost lipid feedstocks. Supercrit. Fluids 2010, 54, 53–60, 10.1016/j.supflu.2010.04.001.
- Thangaraj, B.; Solomon, P.-R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23, 10.1093/ce/zky020.
- Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562, 10.3390/catal8110562
- Lee, J.; Jung, J.-M.; Oh, J.-I.; Ok, Y.S.; Lee, S.-R.; Kwon, E.E. Evaluating the effectiveness of various biochars as porous media for biodiesel synthesis via pseudo-catalytic transesterification. Bioresour. Technol. 2017, 231, 59–64, 10.1016/j.biortech.2017.01.067
- Melani, N.B.; Tambourgi, E.B.; Silveira, E. Lipases: From Production to Applications. Sep. Purif. Rev. 2019, 49, 143–158, 10.1080/15422119.2018.1564328
- Maldonado, R.R.; Lopes, D.B.; Aguiar-Oliveira, E.; Kamimura, E.S.; Macedo, G.A. A review on geotrichum lipases: Production, purification, immobilization and applications. Chem. Biochem. Eng. 2016, 30, 439–454, 10.15255/CABEQ.2016.907
- Alzuhair, S.; Ling, F.W.; Limsong, J. Proposed kinetic mechanism of the production of biodiesel from palm oil using lipase. Process Biochem. 2007, 42, 951–960, 10.1016/j.procbio.2007.03.002
- Sugihara, A.; Tani, T.; Tominaga, Y. Purification and characterization of a novel thermostable lipase from Bacillus sp. J. Biochem. 1991, 109, 211–216, 10.1093/oxfordjournals.jbchem.a123363
- Lanser, A.C.; Manthey, L.K.; Hou, C.T. Regioselectivity of new bacterial lipases determined by hydrolysis of triolein. Curr. Microbiol. 2002, 44, 336–340, 10. I007/s00284-00 1-00 19-3
- Tsurumura, T.; Tsuge, H. Substrate selectivity of bacterial monoacylglycerol lipase based on crystal structure. J. Struct. Funct. Genom. 2014, 15, 83–89, 10.1007/s10969-014-9181-2
- Li, P.-Y.; Zhang, Y.-Q.; Zhang, Y.; Jiang, W.-X.; Wang, Y.-J.; Zhang, Y.-S.; Sun, Z.-Z.; Li, C.-Y.; Zhang, Y.-Z.; Shi, M.; Song, X-Y.; Zhao, L-S.; Chen, X-L. Study on a Novel Cold-Active and Halotolerant Monoacylglycerol Lipase Widespread in Marine Bacteria Reveals a New Group of Bacterial Monoacylglycerol Lipases Containing Unusual C(A/S)HSMG Catalytic Motifs. Frontiers Microbiol. 2020, 11, 9, 10.3389/fmicb.2020.00009
- Götz, F.; Verheij, H.M.; Rosenstein, R. Staphylococcal lipases: molecular characterisation, secretion and processing. Chem. Phys. Lipids 1998, 93, 15-25, 10.1016/S0009-3084(98)00025-5
- Jaeger, K.-E.; Ransac, S.; Dijkstra, B.W.; Colson, C.; Heuvel, M.; van Misset, O. Bacterial lipases. FEMS Microbiol. Rev. 1994, 15, 29–63, 10.1111/j.1574-6976.1994.tb00121.x
- Wei, L.; Li, R.W.; Qiang, L.; Wei, D.; Liu, D.H. Acyl migration and kinetics study of 1(3)-positional specific lipase of Rhizopus oryzae-catalyzed methanolysis of triglyceride for biodiesel production. Process Biochem. 2010, 45, 1888–1893, 10.1016/j.procbio.2010.03.034
- Sánchez, D.A.; Tonetto, G.M.; Ferreira, M.L. Burkholderia cepacia lipase: A versatile catalyst in synthesis reactions. Biotechnol. Bioeng. 2018, 115, 6–24, 10.1016/j.cej.2014.09.088.
- Encinar, J.M.; González, J.F.; Sánchez, N.; Nogales-Delgado, S. Sunflower oil transesterification with methanol using immobilized lipase enzymes. Bioprocess Biosyst. Eng. 2019, 42, 157–166, 10.1007/s00449-018-2023-z
- Riyadi, F.A.; Alam, M.Z.; Salleh, M.N.; Salleh, H.M. Optimization of thermostable organic solvent-tolerant lipase production by thermotolerant Rhizopus Using solid-state fermentation of palm kernel cake. 3 Biotech 2017, 7, 300, 10.1007/s13205-017-0932-1
- Oliveira, F.; Moreira, C.; Salgado, J.M.; Abrunhosa, L.; Venancio, A.; Belo, I. Olive pomace valorization by Aspergillus species: Lipase production using solid-state fermentation. J. Sci. Food Agric. 2016, 96, 3583–3589, 10.1002/jsfa.7544
- Pandey, N.; Dhakar, K.; Jain, R.; Pandey, A. Temperature dependent lipase production from cold and pH tolerant species of Penicillium. Mycosphere 2016, 7, 1533–1545, 10.5943/mycosphere/si/3b/5
- Calabrò, V.; Ricca, E.; De Paola, M.G.; Curcio, S.; Iorio, G. Kinetics of enzymatic trans-esterification of glycerides for biodiesel production. Bioprocess Biosyst. Eng. 2010, 33, 701–710, 10.1007/s00449-009-0392-z
- Dizge, N.; Aydiner, C.; Imer, D.Y.; Bayramoglu, M.; Tanriseven, A.; Keskinler, B. Biodiesel production from sunflower, soybean, and waste cooking oils by transesterification using lipase immobilized onto a novel microporous polymer. Bioresour. Technol. 2009, 100, 1983–1991, 10.1016/j.biortech.2008.10.008
- Gog, A.; Roman, M.; Tos, M.; Paizs, C.; Dan Irimie, F. Biodiesel production using enzymatic transesterification - Current state and Perspectives. Renew. Energy 2012, 39, 10–16, 10.1016/j.renene.2011.08.007
- Shah, S.; Gupta, M.N. Lipase catalyzed preparation of biodiesel from Jatropha oil in a solvent free system. Process Biochem. 2007, 42, 409–414, 10.1016/j.procbio.2006.09.024
- Li, W.; Du, W.; Liu, D. Rhizopus oryzae IFO 4697 whole cell catalyzed methanolysis of crude and acidified rapeseed oils for biodiesel production in tert-butanol system. Process Biochem. 2007, 42, 1481–1485, 10.1016/j.procbio.2007.05.015
- Kaieda, M.; Samukawa, T.; Kondo, A.; Fukuda, H. Effect of methanol and water contents on production of biodiesel fuel from plant oil catalyzed by various lipases in a solvent-free system. J. Biosci. Bioeng. 2001, 91, 12–15, 10.1016/S1389-1723(01)80103-1
- Li, K.; Wang, J.; He, Y.; Cui, G.; Abdulrazaq, M.A.; Yan, Y. Enhancing enzyme activity and enantioselectivity of Burkholderia cepacia lipase via immobilization on melamine glutaraldehyde dendrimer modified magnetic nanoparticles. Chem. Eng. J. 2018, 351, 258–68, 10.1016/j.cej.2018.06.086
- Kim, S.H.; Kim, S.; Park, S.; Kim, H.K. Biodiesel production using cross-linked Staphylococcus haemolyticus lipase immobilized on solid polymeric carriers. J. Mol. Catal. B Enzym. 2013, 85–86, 10–16, 10.1016/j.molcatb.2012.08.012
- Shah, S.; Sharma, S.; Gupta. M.N. Biodiesel preparation by lipase-catalyzed transesterification of Jatropha oil. Energy Fuels 2004, 18, 154–159, 10.1021/ef030075z
- Ji, Q.; Wang, B.; Tan, J.; Zhu, L.; Li, L. Immobilized multienzymatic systems for catalysis of cascade reactions. Process Biochem. 2016, 51, 1193–1203, 10.1016/j.procbio.2016.06.004
- Handayani, R.; Wahyuningrum, D.; Zulfikar, M.A.; Nurbaiti, S.; Radiman, C.L.; Buchari, R. The synthesis of biodiesel catalyzed by Mucor miehei lipase immobilized onto aminated polyethersulfone membranes. Bioresour. Bioprocess 2016, 3, 22, 10.1186/s40643-016-0098-4
- Costa Rodrigues, R.; Volpato, G.; Ayub, M.A.Z.; Wada, K. Lipase-catalysed ethanolysis of soybean oil in a solvent-free system using central composite design and response surface methodology. J. Chem. Technol. Biotechnol. 2008, 83, 849–854, 10.1002/jctb.1879
- Ashjari, M.; Garmroodi, M.; Asl, F.A.; Emampour, M.; Yousefi, M.; Lish, M.P.; Habibi, Z.; Mohammadi, M. Application of multi-component reaction for covalent immobilization of two lipases on aldehyde-functionalized magnetic nanoparticles; production of biodiesel from waste cooking oil. Process Biochem. 2020, 90, 156–167, 10.1016/j.procbio.2019.11.002
- Chen, G.; Ying, M.; Li, W. Enzymatic conversion of waste-cooking oils into alternative fuel biodiesel. Appl. Biochem. Biotechnol. 2006, 132, 911–921, 10.1385/ABAB:132:1:911
- Arumugam, A.; Ponnusami, V. Production of biodiesel by enzymatic transesterification of waste sardine oil and evaluation of its engine performance. Heliyon 2017, 3,e00486, 10.1016/j.heliyon.2017.e00486
- Lv, L.; Dai, L.; Du, W.; Liu, D. Effect of water on lipase NS81006-catalyzed alcoholysis for biodiesel production. Process Biochem. 2017, 58, 239–244, 10.1016/j.procbio.2017.04.033
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-Lipase as Surface Functionalized NanoBiocatalyst for the Production of Biodiesel Using Waste Cooking Oil as Feedstock: Characterization and Process Optimization. Energies 2020, 13, 177, 10.3390/en13010177
- Kaieda, M.; Samukawa, T.; Matsumoto, T.; Ban, K.; Kondo. ; Shimada. Y.; Noda, H.; Nomoto, F.; Ohtsuka, K.; Izumoto, E.; Fukuda, H. Biodiesel fuel production from plant oil catalyzed by Rhizopus oryzae lipase in a water-containing system without an organic solvent. J. Biosci. Bioeng. 1999, 88, 627–631, 10.1016/S1389-1723(00)87091-7
- Duarte, S.H.; Hernández, G.L.P.; Canet, A.; Benaiges, M.D.; Maugeria, F.; Valero, F. Enzymatic biodiesel synthesis from yeast oil using immobilized recombinant Rhizopus oryzae. Bioresour. Technol. 2015, 183, 175–180, 10.1016/j.biortech.2015.01.133
- Shahedi, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; As’habi, M.A. Co-immobilization of Rhizomucor miehei lipase and Candida antarctica lipase B and optimization of biocatalytic biodiesel production from palm oil using response surface methodology. Renew. Energy 2019, 141, 847–857, 10.1016/j.renene.2019.04.042
- Matsuda, T.; Marukado, R.; Mukouyama, M.; Harada, T.; Nakamura, K. Asymmetric reduction of ketones by Geotrichum candidum: Immobilization and application to reactions using supercritical carbon dioxide. Tetrahedron Asymmetry 2008, 19, 2272–2275, 10.1016/j.tetasy.2008.09.018
- Adewale, P.; Dumont, J.-M.; Ngadi, M. Enzyme-catalyzed synthesis and kinetics of ultrasonic assisted methanolysis of waste lard for biodiesel production. Chem. Eng. J. 2016, 284, 158–165, 10.1016/j.cej.2015.08.053
- Wang, L.; Liu, X.; Jiang, Y.; Liu, P.; Zhou, L.; Ma, L.; He, Y.; Li, H.; Gao, J. Silica Nanoflowers-Stabilized Pickering Emulsion as a Robust Biocatalysis Platform for Enzymatic Production of Biodiesel. Catalysts 2019, 9, 1026, 10.3390/catal9121026
- Antonio, D.C.; Amancio, L.P.; Rosset, I.G. Biocatalytic Ethanolysis of Waste Chicken Fat for Biodiesel Production. Catal. Letters 2018, 148, 3214–3222, 10.1007/s10562-018-2529-7
- Lara, P.V.; Park, E.Y. Potential application of waste activated bleaching earth on the production of fatty acid alkyl esters using Candida cylindracea lipase in organic solvent system. Enzyme Microb. Technol. 2004, 34, 270–277, 10.1016/j.enzmictec.2003.10.015
- Matinja, A.I.; Zain, N.A.M.; Suhaimi, M.S.; Alhassan, A.J. Optimization of biodiesel production from palm oil mill effluent using lipase immobilized in PVA alginate- sulfate beads. Renew. Energy 2019, 135, 1178–1185, 10.1016/j.renene.2018.12.079
- Lee, J.H.; Kim, S.B.; Yoo, H.Y.; Lee, J.H.; Han, S.O.; Park, C.; Kim, S.W. Co-immobilization of Candida rugosa and Rhyzopus oryzae lipases and biodiesel production. Korean J. Chem. Eng. 2013, 30, 1335–1338, 10.1007/s11814-013-0058-z
- Cubides-Roman, D.C.; Perez, V.H.; de Castro, H.F.; Orrego, C.E.; Giraldo, O.H.; Silveira, E.G.; David, G.F. Ethyl esters (biodiesel) production by Pseudomonas fluorescens lipase immobilized on chitosan with magnetic properties in a bioreactor assisted by electromagnetic field. Fuel 2017, 196, 481–487, 10.1016/j.fuel.2017.02.014
- Bandikari, R.; Qian, J.; Baskaran, R.; Liu, Z.; Wu, G. Bio-affinity mediated immobilization of lipase onto magnetic cellulose nanospheres for high yield biodiesel in one time addition of methanol. Bioresour.Technol. 2018, 249, 354–360, 10.1016/j.biortech.2017.09.156
- Issariyakul, T.; Kulkarni, M.G.; Dalai, A.K.; Bakhshi, N.N. Production of biodiesel from waste fryer grease using mixed methanol/ethanol system. Fuel Process Technol. 2007, 88, 429–436, 10.1016/j.fuproc.2006.04.007
- Toldrá-Reig, F.; Mora. L.; Toldrá, F. Developments in the use of lipase transesterification for biodiesel production from animal fat waste. Sci. 2020, 10, 5085, 10.3390/app10155085
- Wang, X.; Qin, X.; Li, D.; Yang, B.; Wang, Y. One-step synthesis of high-yield biodiesel from waste cooking oils by a novel and highly methanol-tolerant immobilized lipase. Bioresour. Technol. 2017, 235, 18–24, 10.1016/j.biortech.2017.03.086
- Al-Zuhair, S.; Hasan, M.; Ramachandran, K. Kinetics of the enzymatic hydrolysis of palm oil by lipase. Process Biochem. 2003, 38, 1155–1163, 10.1016/S0032-9592(02)00279-0
- Chesterfield, D.M.; Rogers, P.L.; Al-Zaini, E.O.; Adesina, A.A. Production of biodiesel via ethanolysis of waste cooking oil using immobilised lipase. Chem. Eng. J. 2012, 207, 701–710, 10.1016/j.cej.2012.07.039
- Hwang, H.T.; Qi, F.; Yuan, C.; Zhao, X.; Ramkrishna, D.; Liu, D.; Varma, A. Lipase-Catalyzed Process for Biodiesel Production: Protein Engineering and Lipase Production. Biotechnol. Bioeng. 2014, 111, 639, 10.1002/bit.25162
- Poppe, J.K.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Záchia Ayub, M.A. Enzymatic reactors for biodiesel synthesis: Present status and future prospects. Biotechnol. Adv. 2015, 33, 511-525, 10.1016/j.biotechadv.2015.01.011
- Nielsen, P.M. Production of Fatty Acid Alkyl Esters. World Patent WO/2012/098114, 26 July 2012.
- Fraga, F.C.; Valério, A.; de Oliveira, V.A.; Di Luccio, M.; de Oliveira, D. Effect of magnetic field on the Eversa®Transform 2.0 enzyme: Enzymatic activity and structural conformation. Int. J. Biol. Macromol. 2019, 122, 653–658, 10.1016/j.ijbiomac.2018.10.171
- Viesel. Available online: https://gstarbio.com/es/ (accessed on 25 June 2020).
- Chen, X.; Li, L.; Deng, L.; Pedersen, J.N.; Li, L.; Guo, Z.; Cong, F.; Xu, X. Biodiesel Production Using Lipases. In Lipid Modification by Enzymes and Engineered Microbes; Bornscheuer, U.T., Ed.; Academic Press/AOCS Press: London, UK, 2018, pp. 343–373.
- Kotrba, R. Realizing the Vision to Reclaim, Recycle, Refuel. Biodiesel Magazine. 2015. Available online: http://www.biodieselmagazine.com/articles/265452/realizing-the-vision-to-reclaim-recycle-refuel Retrieved 2020-6-22
- Cui, J.D.; Jia, S.R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28, 10.3109/07388551.2013.795516.
- Wancura, J.H.C.; Rosset, D.V.; Brondani, M., Mazutti, M.A.; de Oliveira, J.V.; Tres, M.V.; Jahn, S.L. Soluble lipase-catalyzed synthesis of methyl esters using a blend of edible and nonedible raw materials. Bioprocess Biosyst. Eng. 2018, 41, 1185–1193, 10.3109/07388551.2013.795516.
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, A.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380, 10.1039/C9CY00415G
- Sobhi, B. Modified-Immobilized Enzymes of High Tolerance to Hydrophilic Substrates in Organic Media; US Patent US9,068,175 B2, 30 June 2015.
- Enzymocore. Available online: https://enzymocore.com/about-us/our-enzymatic-technology. Retrieved 2020-6-22.
