Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Infectious diseases caused by microorganisms are a major threat to human health, mostly because of drug resistance, multi-drug resistance and extensive-drug-resistance phenomena to microbial pathogens. During the past, obtaining hybrid azaheterocyclic drugs represents a powerful and attractive approach in modern antimicrobial therapy with very promising results including overcoming microbial drug resistance.
- hybrid compounds
- antimicrobial
- pyridine
- quinoline
1. Introduction
According to the WHO, infectious diseases caused by microorganisms represent a major threat that affects society and human health, exerting great pressure on health systems, individuals and communities [1]. In particular, overconsumption and widespread use and misuse of antimicrobial agents have resulted in the emergence of drug resistance, multi-drug resistance and extensive-drug-resistance phenomena to microbial pathogens and many other drawbacks (toxicity and non specificity of drugs, high prices, etc.). So far, searching for new chemical entities with improved antimicrobial properties remains a very challenging and important task in medicinal chemistry.
During the last few years, molecular hybridization represents a powerful tool in drug design, by merging two or more drug pharmacophores in a single hybrid multi-functional molecule. Usually, the resulting hybrid entity has superior properties compared with conventional classic drugs, with dual or multiple target mechanisms, better biological activity and specificity, less side effects and toxicity, less drug–drug interactions, etc. [2][3]. As a result of this approach, important advances have been achieved in antimicrobial therapy, some of the present drugs from the market have a hybrid structure and some hybrid structures are in different clinical trials [2][3][4][5][6][7][8].
A literature survey revealed that azines are privileged scaffolds in current medicinal chemistry and drug discovery, possessing a large variety of biological activities, such as: antibacterial, antifungal, antiplasmodial and antimalarial, anthelmintic, antitubercular, antiviral, anticancer, anti-inflammatory, antihypertensive, diuretic, antithrombic, anticoagulant, antidepressant, anxiolytic, anticonvulsant, analgesic, antiulcer, antidiabetic, antihistaminic, etc. [4][5][6][7][8]. As a matter of fact, the greatest majority of the existing drugs from the market contain in their structure a nitrogen heterocycle, some of them being a hybrid structure, which justifies the demand of the pharmaceutical industry for such drugs with nitrogen heterocycle skeleton.
2. Six-Member Ring Azaheterocycles with One Nitrogen Atom. Hybrid Pyridine
In their attempt to identify new antimicrobial compounds, Eryılmaz et al. [9] designed and synthesized different hybrid pyridine derivatives bearing in the 2- and 4-position of the ring of a thiazole moiety. The synthesis was straight and efficient, involving a Hantzsch cyclocondensation of pyridine-2- and 4- carbothioamide 1 and 3 with acetophenone derivatives, when the desired hybrid 4-(R-2-yl)-2-(pyridin-2-yl)thiazole 2a–e and 4-(R-2-yl)-2-(pyridin-4-yl)thiazole 4a–e are obtained, Scheme 1. The synthesized compounds were tested for their antibacterial activity [four strains, Gram-positive (Bacillus cereus, Staphylococcus aureus) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa)] and antifungal activity (one strain, Candida albicans) via minimal inhibitory concentration (MIC) method and DNA cleavage activity studies. The researchers established interesting correlation structure-biological activity (SAR), the most relevant finding being that 4-pyridine thiazole hybrid compounds 4a–e showed more potent activity than 2a–e. The most promising compound was found to be 4c (MIC values 0.01 mM) exhibited on the bacterial strains Staphylococcus aureus and Bacillus cereus.
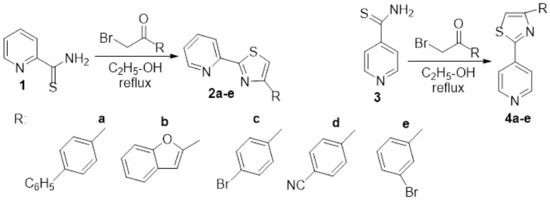
Scheme 1. Reaction pathway to obtain hybrid thiazole-pyridine 2a–e and 4a–e.
In a subsequent paper, some of the above researchers (Cinarli et al. [10]) synthesized different hybrid aroylhydrazone-pyridine-metal derivatives. The newly hybrid aroylhydrazone-pyridine metal derivatives [ZnL2] 7 have been synthesized in two steps: an initial cyclocondensation of pyridine-2-acyl derivative 5 (with aroylhydrazone leading to pyridine-aroylhydrazone ligand 6) is followed by complexation with M2+ metal (Zn2+), Scheme 2.
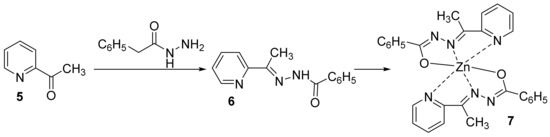
Scheme 2. Reaction pathways to obtain hybrid metal-pyridine derivatives 7.
The synthesized compounds were tested for their antibacterial activity (four strains, Pseudomonas aeruginosa, Escherichia coli, Bacillus cereus and Staphylococcus aureus) and antifungal (one strain, Candida albicans) activity via minimal inhibitory concentration method. The [ZnL2] 7 has been found to be more active than pyridine-aroylhydrazone ligand 6 in all microorganisms (MIC = 11.71 μg/mL for bacteria and MIC = 23.43 μg/mL for C. albicans). The researchers claim that the synthesized new complex acts on microorganisms by disrupting the cell wall structure. The DNA binding interactions was also determined experimentally by spectrophotometric and electrochemical methods. The obtaining data indicate that ligand 6 and hybrid [ZnL2] 7 interact the most with guanine base, and charge transfer is from DNA guanine bases to the molecular structures. Moreover, antioxidant activity was determined, and the hybrid [ZnL2] 7 acted as a scavenger against peroxide radicals.
Trotsko et al. [11] designed and synthesized different hybrid pyridine derivatives bearing at the 2-, 3- or 4- position of the ring of a thiazolidine-2,4-dione moiety. The synthesis involve a condensation reaction of hydrazonyl-pyridine 8a–c with the corresponding (2,4-dioxo-1,3-thiazolidin-5-yl/ylidene) 9a,b/10a–c, which are leading to the desired hybrid pyridine-2,4-dioxo-1,3-thiazolidin-5-yl derivatives 11a–f or pyridine-2,4-dioxo-1,3-thiazolidin-5-ylidene derivatives 12a–i, Scheme 3.
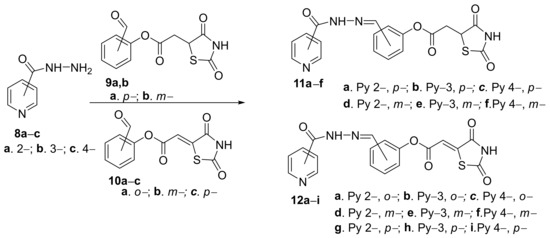
Scheme 3. Reaction pathway to obtain hybrid thiazolidine-pyridine 11a–f and 12a–i.
The in vitro antimycobacterial assay (Mycobacterium tuberculosis) of the newly obtained compounds reveals strong activity in the concentration range of 1–512 μg/mL and low cytotoxicity. Interesting SAR correlations have been performed, and the highest antimycobacterial activity (MIC = 1 μg/mL) was demonstrated for the hybrid pyridine derivatives bearing the thiazolidine-2,4-dione moiety at the 4-position of the pyridine ring (hybrids 11a–c and 12g–i).
Sanad et al. [12] have performed an interesting study concerning the in vitro antimicrobial activity of some newly hybrid thieno-pyrimidin-pyridine derivatives. The synthesized compounds belonged to different classes of substituted pyridine: thiophen-dihydropyridine 14, thiophen-pyrido-pyrimidin-4(1H)-one 15, and fused pyridine: pyrido-thiophen-triazolo-pyrimidine 16a–c, thiophen-pyrido-thieno derivative 17, thiophen-pyrido-thieno-pyrimidin-4-one 18, thiophen-pyrido-thieno-pyrimidin-2,4-dione 19, thiophen-pyrido-thieno-pyrimidin-2-R-4-one 20, Scheme 4.
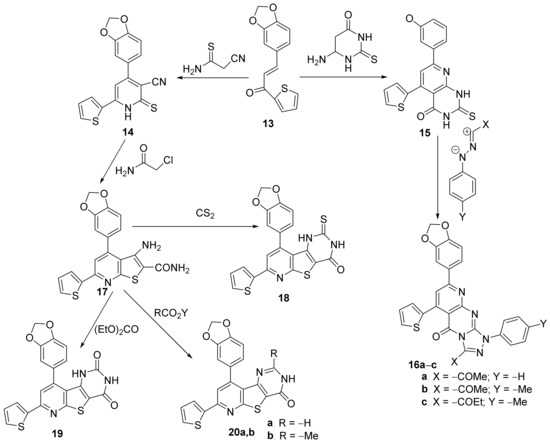
Scheme 4. Reaction pathway to obtain hybrid thiophen-pyrimidin-pyridine 14–20.
The synthetic approach is straight and efficient, involving typical organic chemistry reactions, mostly cyclocondensations. The synthesized compounds were tested in vitro for their antibacterial activity against Escherichia coli and Klebsiella pneumoniae as Gram-negative bacterial strains as well as against Staphylococcus aureus and Streptococcus mutans as Gram-positive bacterial strains. The obtained results (expressed as the diameter of inhibition zones (DIZ) and MIC) reveal that the thiophen-pyrido-thieno-pyrimidin-2-R-4-one 20a,b exhibit the strongest antibacterial activities against all the tested bacteria, in the range of 40–60 mm for inhibition zones, respectively, 4–16 μg/mL for MIC values.
Desai et al. [13] have studied the in vitro antimicrobial activities of some newly hybrid oxazino-pyridine derivatives. The desired compounds, oxazin-3(4H)-yl)phenyl)ethyildene)amino)-6-((arylidene)amino)-4-(4-chlorophenyl)-2-oxo-1,2-dihydropyridine 23a–j, were synthesized in two steps, by cyclocondensation of oxazine 21 followed by condensation of the intermediate 22, Scheme 5.
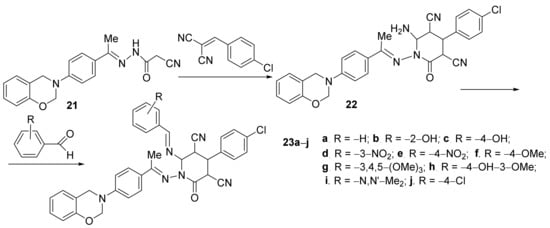
Scheme 5. Reaction pathway to obtain hybrid oxazino-pyridine 23a–j.
The synthesized hybrid compounds were tested for their in vitro antibacterial activity against various bacteria (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pyogenes) and fungus (Candida albicans, Aspergillus niger, Aspergillus clavatus) via the MIC method. Some compounds have proved to have a very powerful activity against bacteria E. coli (23h, MIC = 25 μg/mL) and against fungus C. albicans (23f, MIC = 50 μg/mL), respectively, A. clavatus (23h, MIC = 25 μg/mL).
Sribalan et al. [14] have studied thein vitroantimicrobial activity of some tetrazole-heterocycle hybrid derivatives. The synthesis supposes a cyclocondensation reaction of amide precursors 24 with sodium azide, when the corresponding tetrazolo-pyridine 25a–d and tetrazolo-quinoline 26a–e hybrids are obtained, Scheme 6.
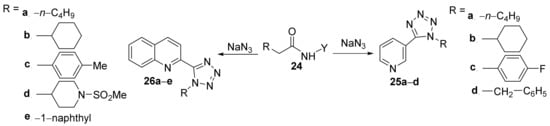
Scheme 6. Reaction pathway to obtain the tetrazolo-pyridine 25a–d and tetrazolo-quinoline 26a–e hybrids.
The synthesized tetrazolo-pyridine 25a–d and tetrazolo-quinoline 26a–e hybrids were tested for their in vitro antibacterial activity against various bacteria (Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pyogenes) and the fungus Candida albicans. An interesting SAR correlation has been performed. The compound 25a (the pyridyl ring is decorated with n-butyl) proved to be the most active from the tetrazolo-pyridine series against all bacteria (DIZ in the range of 4–15 mm), having a superior inhibition to the standard drug (amikacin). The compound 26d (the quinoline ring is decorated with a piperidyl-sulfonamide moiety) proved to be the most active from the tetrazolo-quinoline series against all bacteria (DIZ in the range of 4–10 mm), having a comparable inhibition to the standard. The antifungal activity was negligible.
Kuthyala et al. [15] have studied the in vitro antimicrobial activity of some oxadiazolo-imidazopyridine hybrid derivatives. The synthesis was straight, involving a cyclocondensation reaction of hydrazonyl-imidazopyridine 27 with different benzoic acids, when the corresponding oxadiazolo-imidazopyridine hybrids 28a–j were obtained, Scheme 7.
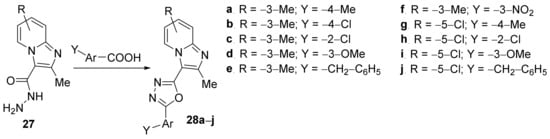
Scheme 7. Reaction pathway to obtain oxadiazolo-imidazopyridine hybrids 28a–j.
The synthesized oxadiazolo-imidazopyridine hybrids 28a–j were tested for their in vitro antibacterial activity against various human bacterial pathogens (Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Bacillus subtilis) and the fungus Candida albicans and Aspergillus niger. An interesting SAR correlation has been performed. The compounds 28f and 28g have high activity against Gram-positive bacteria S. aureus (MIC = 3.12 μg/mL), while compound 28f proved to have high activity against fungus C. albicans (MIC = 12.5 μg/mL).
Ahirwaret al. [16] synthesized two new series of some 1,3,4-triazolo-pyridine hybrid derivatives and studied their antimicrobial activities. The synthesis was conducted in two steps: a cyclocondensation reaction of dithiocarbazate 29 with ammonia leading to the first class of hybrids triazolo-pyridine 30a–n, then an alkylation reaction of 30a–n with benzyl halide takes place leading to the second class of hybrids triazolo-pyridine 31a–n, Scheme 8.
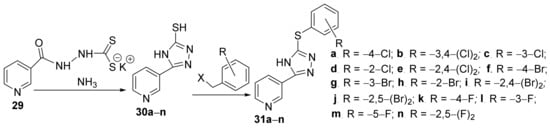
Scheme 8. Reaction pathway to obtain 1,3,4-triazolo-pyridine hybryds 30a–m and 31a–m.
The synthesized triazolo-pyridine hybrids 30a–n and 31a–n were evaluated for their in vitro antibacterial activity against Gram-positive bacteria (three strains: Staphylococcus aureus, Streptococcus pyogenes, Enterococcus faecalis) and Gram-negative bacteria (three strains: Escherichia coli, Pseudomonas aeruginosa, Acinetbacter baumannii) by MIC assay. From the tested compounds, two of them, 31h and 31i, have excellent activity against all strains (MIC in the range of 0.91–11 μg/mL).
Jaabil et al. [17] have studied the in vitro antimicrobial activities of some newly hybrid 1,2,3-triazolo-pyridine derivatives. The synthesis was green and efficient, under grinding strategy at room temperature, involving one-pot sequential multicomponent reactions of aryl aldehydes 32a–r, malonitrile 33, methanol and 1,2,3-triazolyl ketone 34, when the corresponding 1,2,3-triazolyl-pyridine/cyanopyridine hybrids 35a–r were obtained, Scheme 9.
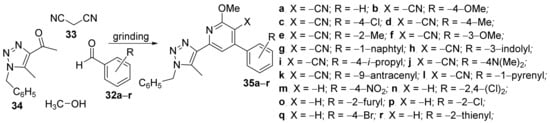
Scheme 9. Reaction pathway to obtain 1,2,3-triazolo-pyridine hybrids 35a–r.
The synthesized 1,2,3-triazolo-pyridine hybrids 35a–r were screened for their in vitro antibacterial activity against three human bacterial strains, Staphylococcus aureus, Salmonella typhi and Escherichia coli, using the MIC method. Some of the 1,2,3-triazolyl cyanopyridine hybrids displayed a remarkable activity against the tested germs, better than tetracycline (standard drug), according to the R-substituent from the phenyl ring. The most active compounds were 35c (with R = −4-chloro-; MIC in the range of 50–90 μg/mL), 35e (with R = −2-methyl-; MIC in the range of 40–90 μg/mL) and 35r (with R = −2-thienyl; MIC in the range of 70–120 μg/mL). The hybrid 1,2,3-triazolo-pyridine compounds were also tested for their antioxidant activity in the assay by 2,2-diphenyl-1-picrylhydrazyl (DPPH) method, showing promising results.
Felefel et al. [18] synthesized three new series of some pyridine hybrid derivatives (namely pyrazole-pyridine 37–41, triazolo-pyridine 42–45 and triazino-pyridine 46) and studied their antimicrobial activities. The synthesis is using as starting material 6-(3,4-dimethylphenyl)-2-hydrazinyl-4-(thiophen-2-yl)-pyridine-3-carbonitrile 36 which react with different compounds with methylene active group (namely acetyl acetone, diethylmalonate, ethyl cyanoacetate, ethyl benzoylacetate and/or ethyl acetoacetate) to produce the desired pyrazole-pyridine hybrid derivatives 37–41, Scheme 10.
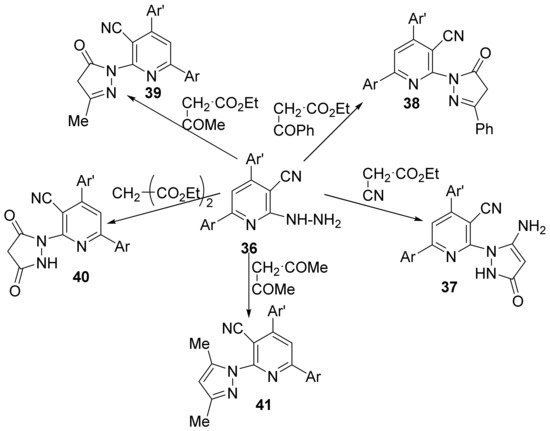
Scheme 10. Reaction pathway to obtain pyrazole-pyridine hybrids 37–41.
The synthesis of triazolo-pyridines 42–45 and tetrazolo-pyridines 46 use as starting material the same intermediate, the 6-(3,4-dimethylphenyl)-2-hydrazinyl-4-(thiophen-2-yl)-pyridine-3-carbonitrile 36, which react with the appropriate formic acid, acetic acid, benzoyl chloride, carbon disulfide, respectively, sodium nitrite, to produce the desired hybrid derivatives 42–45 and 46, Scheme 11.
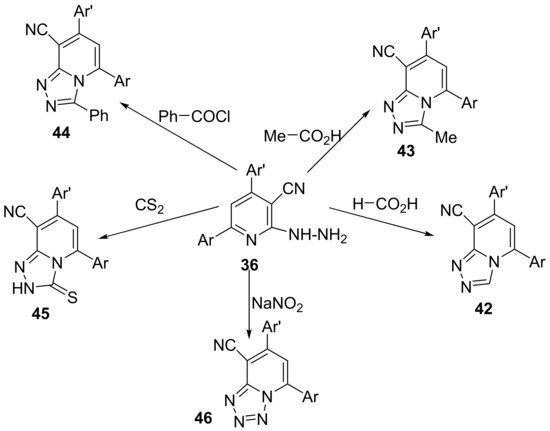
Scheme 11. Reaction pathway to obtain triazolo-pyridines 42–45 and tetrazolo-pyridine 46 hybrids.
The synthesized pyridine hybrids 37–46 were screened for their in vitro antibacterial activity against Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis), Gram-negative bacteria (Salmonella typhi and Escherichia coli) and fungus (Aspergillus flavus and Candida albicans) using the disk diffusion agar technique. Some of the hybrids have significant antimicrobial activity, the most active compounds being 37 with a DIZ in the range of 10–17 mm. The antioxidant activity was also tested.
Amperayani et al. [19] synthesized a library of piperine-pyridine hybrid derivatives and studied their antimicrobial activities. The reaction pathway is straight, in one step, involving an acylation reaction of various amino-pyridine derivatives 47a–h, when the corresponding hybrids piperine-pyridine derivatives 48a–h are obtained, Scheme 12.
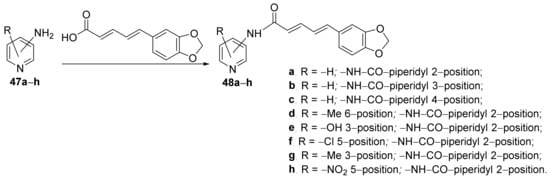
Scheme 12. Reaction pathway to obtain piperine-pyridine hybrids 48a–h.
The synthesized piperine-pyridine hybrid derivatives 48a–h were tested for their in vitro antibacterial activity against some Gram-positive and Gram-negative bacterial strains (Bacillus subtilis, Streptobacillus, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterococcus faecalis and Salmonella typhi) and fungus strains (Aspergillus niger, Aspergillus flavus, Aspergillus fumigatus and Candida albicans) using the disk diffusion agar technique. The piperine-pyridine hybrids 48a, 48d and 48h have very good activity against the Gram-negative strains E. coli, K. pneumoniae, E. faecalis and P. aeruginosa, having a DIZ in the range of 22–26 mm, superior to control standard drug). The antifungal activity of hybrids was moderate.
3. Six-Member Ring Azaheterocycles with One Nitrogen Atom. Hybrid Quinoline and Isoquinoline
In their attempt to obtain new quinoline derivatives with antimicrobial activity, Albayrak et al. [20] synthesized a library of 20 new triazolo-quinoline hybrid derivatives and studied their antimicrobial activities. The reaction pathway involves several steps (Scheme 13), starting from 8-nitroquinoline 53. The initial reduction reaction of 53 is leading to 8-aminoquinoline 54, which is suffering a subsequent N-alkylation with azido-iodo-propane 52a,b (generated from the corresponding bromo-alkyl alcohol) leading to alkyl-azide-quinolines 55 and 56. Finally, the alkyl-azide-quinoline derivatives are treated with the corresponding alkyne 57a–j leading to the desired products, the triazolo-quinoline hybrid derivatives 58a–j and 59a–j.
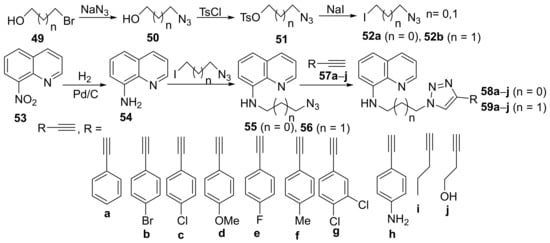
Scheme 13. Reaction pathway to obtain triazolo-quinoline hybrids 58a–j and 59a–j.
The synthesized triazolo-quinoline hybrid derivatives 58a–j and 59a–j were tested for their in vitro antibacterial activity against some Gram-positive and Gram-negative bacterial strains (Bacillus subtilis, Streptococcus pneumoniae, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Enterococcus faecalis) and fungus strains (Candida parapsilosis and Candida albicans) using the disk diffusion agar technique. The triazolo-quinoline hybrid derivatives 58a–j and 59a–j manifest good activity against the tested strains. The most active compound was 58a, having excellent activity against E. coli, P. aeruginosa, K. pneumoniae, E. faecalis, S. aureus, S. pneumoniae, B. subtilis, C. albicans and C. parapsilosis. In some cases, the activity was several orders of magnitude superior to control drugs (DIZ of 58a was in the range of 35–250 mm; control, ampicillin, respectively, fluconazole have had a DIZ of 35 mm).
Hryhoriv et al. [21][22] synthesized two new classes of hybrid derivatives analogous to fluoroquinolones, namely piperidino-quinoline 61a,b and 1,2,3-triazolo-piperidino- quinoline 62a–k, and studied their antimicrobial activities. The first class of hybrids was obtainedviaan N-alkylation reaction of piperidino-quinoline 60a,b, when the N-substituted-piperidino-quinoline hybrids 61a,b are obtained. A click cyclocondensation reaction of 61a,b occurs to the second class of hybrids, the 1,2,3-triazolo-piperidino-quinoline 62a–k, Scheme 14.
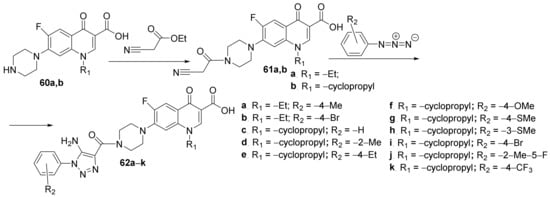
Scheme 14. Reaction pathway to obtain N-substituted-piperidino-quinoline hybrids 61a,b and1,2,3-triazolo-piperidino-quinoline hybrids 62a–k.
The synthesized hybrid derivatives piperidino-quinoline 61a,b and 1,2,3-triazolo-piperidino-quinoline 62a–k were tested for their in vitro antibacterial activity against standard bacterial strains Staphylococcus aureus and Escherichia coli, respectively, and the fungus Candida albicans using the disk diffusion agar technique. The antimicrobial assay was also made by some clinical bacterial strains S. aureus and E. coli, respectively, and fungus C. albicans using the same method. The hybrid, 1,2,3-triazolo-piperidino-quinoline 62c have a very good activity against the tested standard strains (DIZ in the range of 25–35 mm), having a superior inhibition zone to control (DIZ = 25 mm). Against clinical microbial strains, the activity was negligible.
Drweesh et al. [23] synthesized hybrid organic-inorganic derivatives and studied their antimicrobial activities, antiproliferative activity, and radical scavenging properties. In order to synthesize the desired palladium-quinoline derivatives 64a–d, they used organic cation modulation, doing a complexation reaction with PdCl2 of the quinolines 63a–d, Scheme 15.
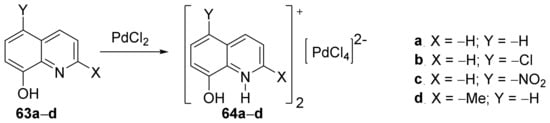
Scheme 15. Reaction pathway to obtain metal-quinoline hybrids 64a–d.
The synthesized palladium-quinoline derivatives hybrids 64a–d and the free ligands 63a–d, were tested for their in vitro antimicrobial activity against 14 standard microbial strains (Gram-positive and Gram-negative bacteria, fungus: Bifidobacterium animalis, Lactobacillus plantarum, Bacillus subtilis, Staphylococcus aureus ATCC 663, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa, Proteus mirabilis , Escherichia coli, Salmonella enterica, Candida albicans, Saccharomyces boulardii, Aspergillus flavus, Trichoderma viridae, Aspergillus niger). All hybrid compounds 64a–d have high antimicrobial activity against all tested strains, with minimum inhibitory concentration values ranging from 1.95 to 250 μg/mL. The results of DNA interaction studies indicate that the hybrids 64a–d and the free ligands 63a–d, interact with the DNAvia an intercalation mechanism (the aromatic chromophore intercalates the base pairs of DNA; compound 64a has the highest binding affinity). The anticancer activity was also studied, with compounds 64a and 64b having selective and high cytotoxicity against human lung and breast cancer cells.
Nehra et al. [24] synthesized a series of triazole-benzothiazole-quinoline hybrids and studied their antimicrobial properties. The reaction pathway is straight and efficient (Scheme 16), involving a click cyclocondensation reaction of azido-alkyl-benzothiazole 65a–f (generated in situ from the corresponding bromo-alkyl derivative) with the corresponding alkyne-quinoline, leading to the desired products, triazole- -benzothiazole-quinoline hybrids 66a–f.
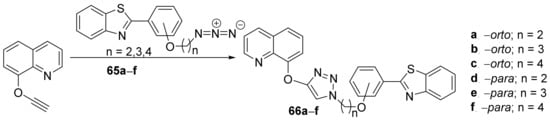
Scheme 16. Reaction pathway to obtain triazole-benzothiazole-quinoline hybrids 66a–f.
The synthesized hybrids 66a–f were evaluated for their in vitro antimicrobial activity against two Gram-positive strains (Staphylococcus aureus and Bacillus subtilis) and two Gram-negative strains (Escherichia coli and Pseudomonas aeruginosa) and two fungal strains (Candida tropicalis and Aspergillus terreus). The tested hybrids have good antimicrobial activity against both bacteria and fungus. The most promising compound was proved to be 66a, with an antibacterial (DIZ in the range of 15–17 mm) and antifungal (DIZ in the range of 21–34 mm) activity superior to reference ciprofloxacin (DIZ = 22 mm) and fluconazole (DIZ = 20 mm), respectively. Interesting molecular docking studies were also performed.
Awolade et al. [25] synthesized a library of triazole-quinoline hybrids and studied their antimicrobial properties. The reaction pathway is straight involving click chemistry of various azides with triple bond derivatives, via copper(I)-catalyzed azide-alkyne 3 + 2 dipolar cycloaddition reactions, Scheme 17.
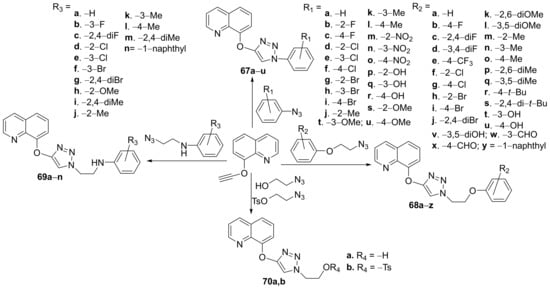
Scheme 17. Reaction pathway to obtaintriazole-quinoline hybrids 67a–u, 68a–z, 69a–n and 70a,b.
The synthesized hybrids 67a–u, 68a–z, 69a–n and 70a,b were evaluated for their in vitro antimicrobial activity against ESKAPE microbial strains (bacteria and fungus: (Staphylococcus aureus, Escherichia coli, Acinetbacter baumannii, Klebsiella pneumoniae, Candida albicans and Candida neoformans). Some of the compounds proved to have a good and broad-spectrum of antibacterial activity, against methicillin-resistant S. aureus (MRSA), E. coli, A. baumannii, multidrug-resistant K. pneumoniae and the fungus C. albicans and C. neoformans (superior to control, fluconazole). The most promising antibacterial compound was proved to be 70b with an MIC = 75.39 μM against MRSA, E. coli, A. baumannii, and multidrug-resistant K. pneumoniae. The hybrid 70b also has a very good antifungal activity against C. albicans and C. neoformans with an MIC of 37.69 and 2.36 μM, respectively, superior to control fluconazole.
Ammar et al. [26] synthesized a series of thiazole-quinoline hybrids and studied their antimicrobial properties. In order to synthesize the desired compounds, they used the condensation reaction between formil-quinoline derivatives with amino-thiazole or sulfathiazole, when the desired Schiff’s base thiazole-quinoline 71 and 72, are obtained, Scheme 18.
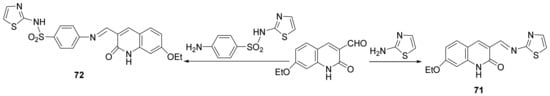
Scheme 18. Reaction pathway to obtain thiazole-quinoline hybrids 71 and 72.
Further, the condensation reaction between formil-quinoline derivatives with different thiazolone derivatives lead to hybrid thiazolone-quinoline derivatives 73–76, Scheme 19.
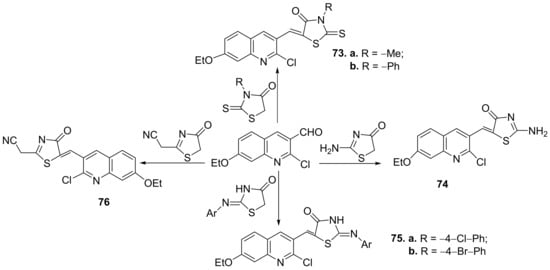
Scheme 19. Reaction pathway to obtain thiazolone-quinoline hybrids 73–76.
Finally, the cyclization of different quinoline-thiosemicarbazone derivatives with the halogenated compounds lead to other hybrid thiazole-quinoline derivatives 77–82, Scheme 20.
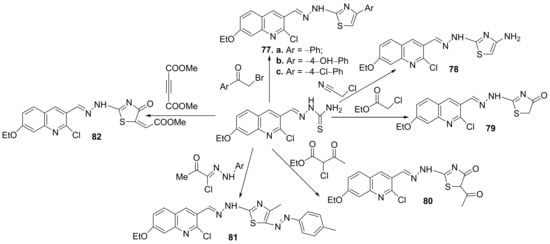
Scheme 20. Reaction pathway to obtain thiazole-quinoline hybrids 77–82.
The synthesized hybrids 71–82, were evaluated for theirin vitroantimicrobial activity against eight standard microbial strains, three Gram-positive bacteria (Staphylococcus aureus, Bacillus faecalis and Bacillus subtilis), three Gram-negative bacteria (Escherichia coli, Salmonella typhi and Pseudomonas aeruginosa), and two fungi (Candida albicans and Fusarium oxysporum). Some of the compounds have good antimicrobial activity, with MIC and MBC values ranging between 0.95 and 62.5 µg/mL, and 1.94 and 118.7 µg/mL, respectively. Two compounds, namely 77b and 73a, proved to be the most active of the series against S. aureus and E. coli having an MIC between 0.95 and 7.81 μg/mL, respectively a MBC between 3.31 and 15.62 μg/mL.
Using a similar strategy, some of the above researchers (Eissa et al. [27]) synthesized a new series of thiazole-quinoline hybrids and studied their antimicrobial properties. In order to synthesize the desired compounds, they used the cyclization of quinoline-thiosemicarbazone derivatives with the halogenated compounds, when the corresponding hybrid thiazole-quinoline derivatives, 83a–f, 84a–f and 85a–f are obtained, Scheme 21.
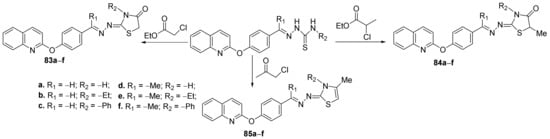
Scheme 21. Reaction pathway to obtain thiazole-quinoline hybrids 83–85a–f.
The synthesized hybrids 83a–f, 84a–f and 85a–f, were evaluated for their in vitro antimicrobial activity against Gram-positive (five strains: Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Bacillus subtilis and Enterococcus faecalis) and Gram-negative bacteria (five strains: Neisseria gonorrhoeae, Proteus vulgaris, Klebsiella pneumonia, Shigella flexneri and Pseudomonas aeruginosa), as well as fungus (five strains: Aspergillus fumigatus, Aspergillus clavatus, Candida albicans, Geotrichum candidum, and Penicillium marneffei). Some of the compounds displayed good antimicrobial activity, superior to the used control. The most active compound was found to be 85e, having a two-fold potency compared with gentamycin for inhibition of N. gonorrhoeae, four-fold potency compared with amphotericin B for the inhibition of A. fumigatus, equipotent activity compared with the reference drugs for inhibition of S. flexneri, S. pyogenes, P. vulgaris, A. clavatus, G. candidum and P. marneffei.
Lagdhir et al. [28] synthesized a library of piperazin-quinoline hybrids and studied their antimicrobial properties. The reaction pathway involves two steps (an alkylation and a condensation reaction), leading to the piperazin-quinoline hybrids 86a–l, Scheme 22.
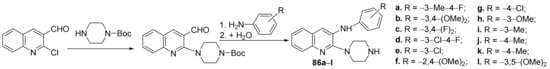
Scheme 22. Reaction pathway to obtain piperazin-quinoline hybrids 86a–l.
The synthesized hybrids 86a–l were evaluated for their in vitro antibacterial (Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli and Pseudomonas aeruginosa) and antifungal (Aspergillus clavatus, Aspergillus niger and Candida albicans) activity, antimalarial (Plasmodium falciparum) and antituberculosis (Mycobacterium tuberculosis) activity. Some of the compounds have good antibacterial and antifungal activity against S. aureus and C. albicans. The hybrids 86a, 86b, 86d, 86j and 86k, are the most active as an antimicrobial against S. aureus, having an MIC = 100 μg/mL, equal to the control drug ampicillin. The hybrid 86k has excellent antifungal activity against C. albicans, having an MIC = 250 μg/mL, two folds higher compared with the control drug griseofulvin. The antimalarial and antitubercular activity proved to be moderate for the majority of compounds.
Desai et al. [29] synthesized a series of pyridine-quinoline hybrids and evaluated it for their antimicrobial properties. The reaction pathway involves a cyclocondensation reaction of quinoline derivative with benzylidene-malononitril, when the corresponding pyridine-quinoline hybrids 87a–j were obtained, Scheme 23.
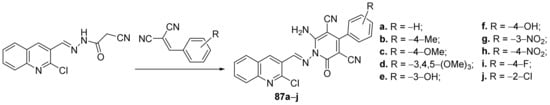
Scheme 23. Reaction pathway to obtain pyridine-quinoline hybrids 87a–j.
The synthesized hybrids 87a–j were evaluated for their in vitro antimicrobial activity against Gram-positive (two strains: Staphylococcus aureus and Staphylococcus pyogenes) and Gram-negative (two strains: Escherichia coli and Pseudomonas aeruginosa) bacteria, as well as to fungus (three strains: Aspergillus clavatus, Aspergillus niger and Candida albicans). Some of the compounds displayed promising antimicrobial activity. The hybrid 87i has the best antibacterial activity against E. coli, P. aeruginosa and S. aureus strains, with an MIC = 12.5 μg/mL, two folds higher compared with the control drug ciprofloxacin (MIC = 25 μg/mL). The most active compound against C. albicans was found to be 87e, having an MIC=25 μg/mL, much better compared with the control drug griseofulvin (MIC = 500 μg/mL).
Vishnuvardhan et al. [30] synthesized a library of triazole-quinoline hybrids and studied their antimicrobial properties. The reaction pathway involves a typical click cyclocondensation reaction of quinoline with a triple bond with aryl-azide derivatives, when the corresponding triazole-quinoline hybrids 88a–l, Scheme 24.
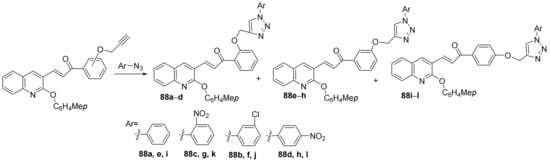
Scheme 24. Reaction pathway to obtain triazole-quinoline hybrids 88a–l.
The synthesized hybrids 88a–l were evaluated for theirin vitroantimicrobial activity against Gram-positive (Staphylococcus aureus and Enterococcus faecalis) and Gram-negative (Escherichia coli and Pseudomonas aeruginosa) bacteria, as well as to fungus (Aspergillus niger and Candida albicans). Most of the hybrid compounds have good antimicrobial activity. The best antibacterial activity reveals the hybrids 88d, 88h and 88i, having a DIZ in the range of 16–21 mm, superior to control ampicillin (DIZ = 15 mm). The best antifungal activity reveals the hybrids 88d, 88h and 88k, having a DIZ in the range of 18–27 mm, superior to control griseofulvin (DIZ = 17 mm).
Abdel-Rahman et al. [31] synthesized a series of piperazin-quinoline hybrids derived from ciprofloxacin and studied their antimicrobial and anticancer properties. The reaction pathway involves the reaction of ciprofloxacin with the corresponding phenolic derivatives with an excess of formaldehyde, when the piperazin-quinoline hybrids 89a–j are obtained, Scheme 25.
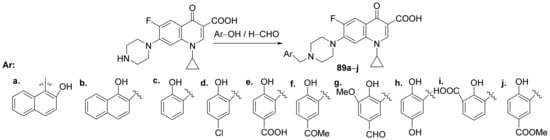
Scheme 25. Reaction pathway to obtain piperazin-quinoline hybrids 89a–j.
The synthesized hybrids 89a–j were evaluated for their antimicrobial and anticancer activity. The antibacterial screening was preconformed on Gram-positive and Gram-negative strains: Staphylococcus aureus, MRSA clinical strain, MRSA reference strain, Escherichia coli and Pseudomonas aeruginosa. The obtained results reveal that the hybrid 89d has the best antibacterial activity against S. aureus, MRSA (reference strain) and MRSA (clinical strain) with an MIC of 0.57, 0.52, and 0.082 µg/mL, respectively, (compared with the reference standard drug ciprofloxacin which has an MIC of 1.63 µg/mL against S. aureus, an MIC of 1.45 µg/mL against MRSA reference, and an MIC of 0.84 µg/mL against MRSA clinical). The hybrid 89j exhibited the best antimicrobial activity against E. coli and P. aeruginosa, with an MIC of 0.036 and 0.043, respectively, (compared with the reference standard drug ciprofloxacin which has an MIC of 0.056 µg/mL against E. coli and an MIC of 1.27 µg/mL against P. aeruginosa).
Mohammed et al. [32] synthesized a series of glycosylated-quinoline hybrids derived from fluoroquinolone and studied their antimicrobial properties. The reaction pathway involves the reaction of ciprofloxacin with the corresponding phenolic derivative with an excess of formaldehyde, when the glycosylated-quinoline hybrids 90–94 are obtained, Scheme 26.
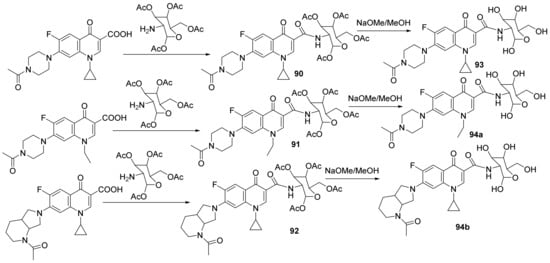
Scheme 26. Reaction pathway to obtainglycosylated-quinoline hybrids 90–94.
The synthesized glycosylated-quinoline hybrids 90–94 were evaluated for their antibacterial activity against various Gram-positive and Gram-negative bacteria: Escherichia coli, Listeria monocytogenes, Salmonella enterica, Pseudomonas aeruginosa, Listeria monocytogenes, E. coli clinical isolate (resistant to nalidixic acid, ciprofloxacin HCl and norfloxacin antibiotics), methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive Staphylococcus aureus (MSSA). The hybrids were also tested for their antifungal activity against fungi: Candida albicans, Aspergillus flavus, Fusarium solani, Stachybotrys chartarum and Penicillium chrysogenum. The hybrid compounds 90, 91 and 94a have excellent antimicrobial activity against a fluoroquinolone-resistant E. coli clinical isolate, comparable to controls ciprofloxacin and norfloxacin. The hybrid compound 91 also has good antifungal activity against C. albicans and P. chrysogenum.
Shruthi et al. [33] synthesized a series of piperazine-quinoline hybrids 95a–e and morpholine-quinoline hybrids 96a–f and evaluate them for their antimicrobial properties. The reaction pathway is depicted in Scheme 27.
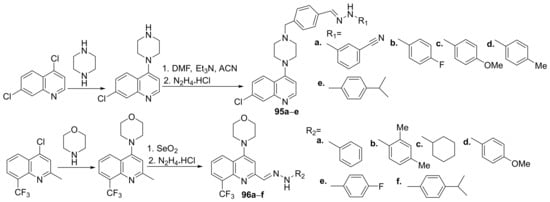
Scheme 27. Reaction pathway to obtain piperazine- and morpholine-quinoline hybrids 95a–e and 96a–f.
The synthesized hybrids 95a–e and 96a–f were evaluated for their antibacterial (Acinetobacter baumanii, Enterococcus faecium, Klebsiella pneumonia, Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus) and antitubercular (Mycobacterium tuberculosis) activity. Hybrid 95b has the best antibacterial activity against E. coli and S. aureus strains with an MIC of 4, respectively, 2 µg/mL, compared to standard drug vancomycin (MIC of 16, respectively, 0.5 µg/mL). Hybrids 95d, 95e and 96f exhibited the best antibacterial activity against A. baumaniistains with an MIC in the range of 1–2 µg/mL, compared to standard drug vancomycin (MIC = 0.5 µg/mL). Hybrids 95b, 95d and 95e also have promising antitubercular activity with an MIC of 4 µg/mL.
Kaur et al. [34] synthesized a series of 3- and 7- substituted-quinoline hybrids derived from fluoroquinolone and studied their antimicrobial properties. The reaction pathway involves the reaction of fluoroquinolone derivatives with the corresponding reagents, when the quinoline hybrids 97–104a,b are obtained, Scheme 28 and Scheme 29.
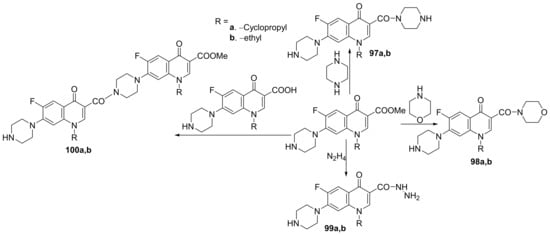
Scheme 28. Reaction pathway to obtain piperazino-quinoline hybrids 97–100a,b.
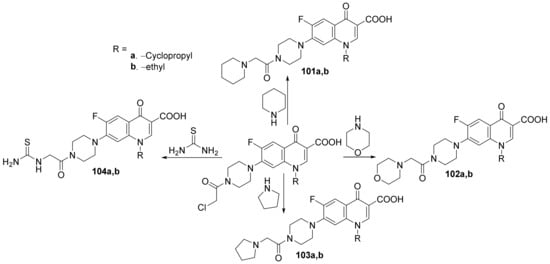
Scheme 29. Reaction pathway to obtain 7-substituted-quinoline hybrids 101–104a,b.
The synthesized quinoline hybrids 97–104a,b were evaluated for their antibacterial activity against four bacterial strains: Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus. All hybrids 97–104a,b have proved to be active against all bacterial strains, with an MIC value of 25 μg/mL which is fourfold more active compared to the standard drug ciprofloxacin (MIC = 100 μg/mL).
Insuasty et al. [35] synthesized a series of imidazolium-quinoline hybrids and studied their antimicrobial properties. The reaction pathway involves the reaction of 3-formyl-quinolone derivatives with the corresponding imidazolium salts, when the imidazolium-quinoline hybrids 105a–h are obtained, Scheme 30.
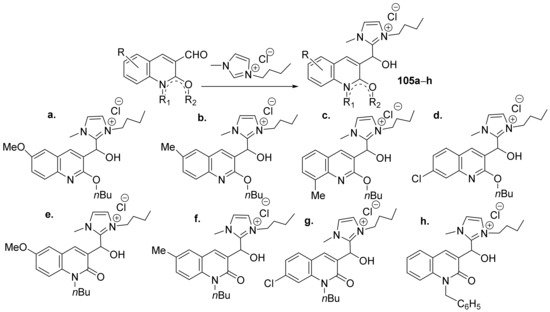
Scheme 30. Reaction pathway to obtain imidazolium-quinoline hybrids 105a–h.
The synthesized imidazolium-quinoline hybrids 105a–h were evaluated for their antibacterial (Klebsiella pneumoniae, Escherichia coli and Staphylococcus aureus), antifungal (Cryptococcus neoformans) and antitubercular (Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG) activities. Hybrid derivatives 105c,d demonstrated a remarkable antifungal activity against C. neoformans (MIC in the range of 15 µg/mL) while for the other fungal strains the activity is weak. The hybrids have modest antibacterial activity (both against Gram-positive and Gram-negative bacteria) as well as antitubercular activity.
Baartzes et al. [36] synthesized a series of benzimidazole-quinoline and ferrocenyl-quinoline hybrids and studied their antimicrobial properties. The reaction pathway involves the reaction of amino-quinoline derivatives with the corresponding formyl derivatives, when the benzimidazole-quinoline hybrids 106a–e and ferrocenyl-quinoline hybrids 107a–e are obtained, Scheme 31.
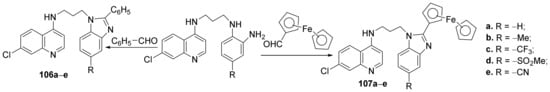
Scheme 31. Reaction pathway to obtain benzimidazole-quinoline and ferrocenyl-quinoline hybrids 106a–e and 107a–e.
The synthesized quinoline hybrids 106a–e and 107a–e were evaluated for their antimalarial (Plasmodium falciparum and Plasmodium berghei) and antitubercular (Mycobacterium tuberculosis) activity. All hybrid derivatives are active against tested malaria strains and have modest activity against them. The most active hybrids against malarial strains have proved to be 106c and 107b, with an IC50 of 0.43, respectively, 0.32 µM, compared with the standard drug chlorquine (IC50 = 0.01 µM).
Fedorowicz et al. [37] synthesized a series of zwiterionic hybrids pyridine-fluoroquinolone 108a–h and quinoline-fluoroquinolone 109a–h and studied their antimicrobial properties. The reaction pathway involves a tandem Mannich-electrophilic amination reaction of isoxazolones derivatives and fluoroquinolone bearing a secondary amino group at position 7 of the quinoline ring, Scheme 32.
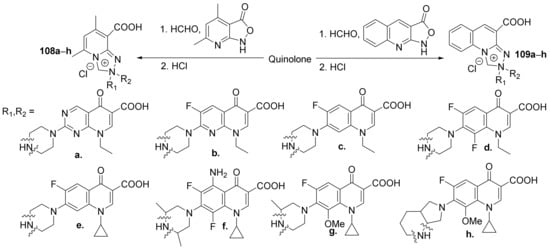
Scheme 32. Reaction pathway to obtain zwiterionic pyridine-fluoroquinolone and quinoline-fluoroquinolone hybrids 108a–h and 109a–h.
The synthesized quinoline hybrids 108a–h and 109a–h were evaluated for their antibacterial activity against Gram-positive and Gram-negative bacterial strains (laboratory and clinical: Staphylococcus aureus ATCC 6538, Staphylococcus aureus MRSA N315, Staphylococcus epidermidis ATCC 14990, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 9027, Proteus vulgaris NCTC 4635, Staphylococcus aureus MRSA 6347, Staphylococcus epidermidis MRSE 13199 and Serratia marcescens 12795) as well as for antibiofilm activity. The hybrid derivatives proved to have bactericidal and antibiofilm activity. The most active hybrids were found to be 109d and 109e, exhibiting good inhibition against all strains, with the IC50 values in the low micromolar range.
Borazjani et al. [38] synthesized a library of quinoline hybrids (benzothiazole-benzo-quinoline 110, imino-benzothiazole-benzo-quinoline 111a–d, β-lactam-benzo-thiazole-benzo-quinoline 112a–m) and studied their antimicrobial properties. The reaction pathway involves a [2+2]-cycloaddition reaction of imines 111a–d and ketenes derived from substituted acetic acids, Scheme 33.
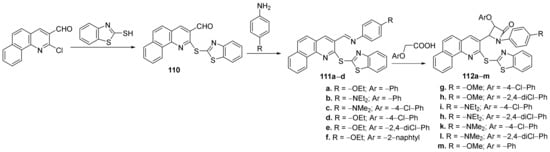
Scheme 33. Reaction pathway to obtain benzothiazole-benzo-quinoline hybrids 110, 111a–d and 112a–m.
The synthesized quinoline hybrids 110–112 were evaluated for their antimicrobial activity against Gram-positive and Gram-negative bacterial strains: Staphylococcus aureus, Bacillus subtilis, Enterococcus faecalis, Salmonella typhi, Escherichia coli and Pseudomonas aeruginosa. From the β-lactam class, the assay indicates that the most active hybrids against E. coli and P. aeruginosa, are 112k and 112m, with an MIC of 42, respectively, 20 μg/mL, compared to standard drug gentamycin (MIC of 90, respectively, 5 μg/mL). From the imino-benzothiazole-benzo-quinoline class, the most active hybrids against P. aeruginosa and S. aureus, are 111a–c, with an MIC of 42 μg/mL, compared to standard drug gentamycin (MIC of 5, respectively, 90 μg/mL).
Berry et al. [39] synthesized a series of peptide-fluoroquinolone hybrids and studied their antimicrobial properties. In order to synthesize the desired hybrids, the researchers used solid-phase peptide synthesis, from levofloxacin fluoroquinolone with the corresponding peptide (oligopeptide), when the desired peptide-fluoroquinolone hybrids 113a–l are obtained, Scheme 34.
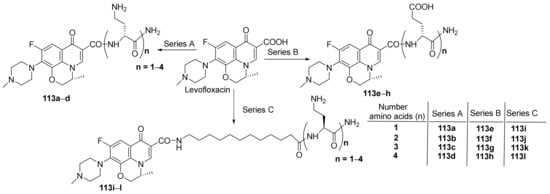
Scheme 34. Reaction pathway to obtain peptide-quinolone hybrids 113a–l.
The synthesized peptide-fluoroquinolone hybrids 113a–l were evaluated for their antimicrobial activity against MDR bacterial strains, Gram-negative and Gram-positive: Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus epidermis (MRSE), Enterococcus faecalis, Enterobacter cloacae, Stenotrophomonas maltophilia. The assay indicates that all the peptide-hybrids have weak antibacterial activity. If the hybrids are mixed with fluoroquinolone (ciprofloxacin, levofloxacin and moxifloxacin) drugs, the resulting conjugates possess antimicrobial activity against MDR Gram-negative bacteria (clinical isolates, P. aeruginosa, E. coli, K. pneumoniae, A. baumannii), superior to reference levofloxacin.
Mermer et al. [40] synthesized a library of triazole- and oxadiazole-fluoroquinolone hybrids and studied their antimicrobial properties. The reaction pathway took placeviaseveral steps of sequential reactions, starting from phenyl piperazine. Finally, the corresponding triazole-fluoroquinolone 114a,b and oxadiazole-fluoroquinolone 115a–j hybrids were obtained via a one-pot three-component Mannich reaction, Scheme 35. The reactions were performed both under conventional thermal heating and microwave, the last pathway being more advantageous.
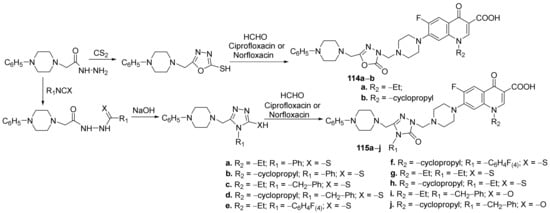
Scheme 35. Reaction pathway to obtain oxadiazole- and triazole-fluoroquinolone hybrids 114a,b and 115a–j.
The synthesized hybrids 114a,b and 115a–j were tested for their antimicrobial activity (against Gram-positive and Gram-negative strains: Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter haemolyticus), DNA gyrase and Topoisomerase IV inhibition potentials. The hybrids have good antimicrobial activity and displayed excellent DNA gyrase inhibition. The hybrids 114b, 115b and 115h exhibited the best antimicrobial activity against the tested strains. Thus, the hybrids have excellent activity against K. pneumoniae with an MIC of 0.25 µg/mL, compared with the standard drug gentamycin (MIC = 0.25 µg/mL). The hybrids also have excellent activity against A. haemolyticus and P. aeruginosa with an MIC in the range of 0.5–2 µg/mL, compared with the standard drug gentamycin (MIC = 0.78 µg/mL, respectively, MIC = 1.56 µg/mL). Against Gram-positive strain E. faecalis the hybrids have excellent activity with an MIC in the range of 0.5–8 µg/mL, compared with the standard drug ampicillin (MIC = 12.5 µg/mL).
Guo et al. [41] synthesized a library of oxadiazole-quinoline hybrids and studied their antibacterial properties. The reaction pathway is straight, involving an alkylation reaction of fluoroquinolone with the corresponding oxadiazole, when the desired oxadiazole-fluoroquinolone hybrids 116a–t were obtained, Scheme 36.
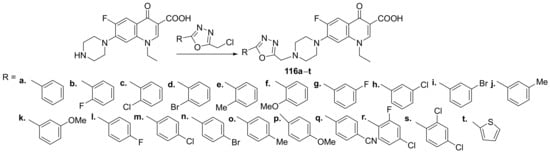
Scheme 36. Reaction pathway to obtain oxadiazole-fluoroquinolone hybrids 116a–t.
The synthesized oxadiazole-fluoroquinolone hybrids 116a–t were tested for their antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) and laboratory Staphylococcus aureus. The hybrids displayed good antibacterial activity, one of the compounds 116k exhibited excellent antibacterial activity against both methicillin-resistant S. aureus and laboratory S. aureus, with an MIC in the range of 0.25–2 μg/mL, superior to control drug vancomycin (MIC = 2 μg/mL).
Wang et al. [42] synthesized a series of benzimidazole–quinoline hybrids and studied their antibacterial and antifungal properties. The reaction pathway involves an N-alkylation reaction of fluoroquinolone with the corresponding benzimidazole, when the desired benzimidazole-fluoroquinolone hybrids 117a–g, 118a,b and 119a–f, were obtained, Scheme 37.
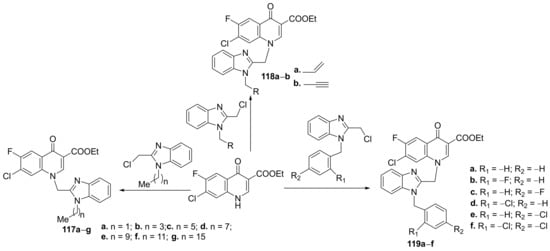
Scheme 37. Reaction pathway to obtain benzimidazole-quinoline hybrids 117a–g, 118a,b and 119a–f.
The synthesized benzimidazole-fluoroquinolone hybrids 117a–g, 118a,b and 119a–f were screened against Gram-positive and Gram-negative bacteria, respectively, fungus (methicillin-resistant Staphylococcus aureus (MRSA), Enterococcus faecalis, Staphylococcus aureus, Staphylococcus aureus ATCC25923, Staphylococcus aureus ATCC29213, Klebsiella pneumonia, Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumanii, Pseudomonas aeruginosa ATCC27853, Escherichia coli ATCC25922, Candida albicans, Candida tropicalis, Aspergillus fumigatus, Candida albicans ATCC90023, Candida parapsilosis ATCC22019). The results of the assay were promising, with some hybrids having excellent antibacterial activity. The most active hybrids against K. pneumonia are 117a and 117c, with an MIC of 8 μg/mL, compared to the standard drug norfloxacin (MIC > 512 μg/mL).The most active hybrids against S. aureus are 119a and 119f, with an MIC of 4 μg/mL, compared to the standard drug norfloxacin (MIC = 64 μg/mL).
Bharadwaj et al. [43] synthesized a series of oxadiazole–quinoline hybrids and studied their antibacterial and antifungal properties. The reaction pathway involves a cyclocondensation reaction of hydrazinyl-quinoline derivative with the corresponding aromatic acids, when the desired oxadiazole–quinoline hybrids 120a–g were obtained, Scheme 38.
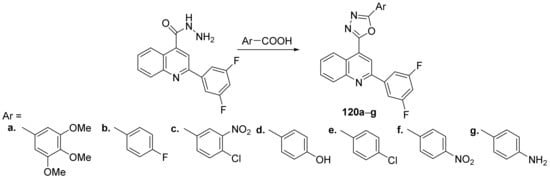
Scheme 38. Reaction pathway to obtain oxadiazole-quinoline hybrids 120a–g.
The synthesized oxadiazole–quinoline hybrids 120a–g were tested against clinical isolates Gram-positive and Gram-negative bacteria (Staphylococcus aureus, Bacillus cereus, Escherichia coli, Serratia marcescens), respectively, fungus (Aspergillus niger, Trichophyton mentagrophytes, Candida albicans, Candida parapsilosis). The antimicrobial activity of oxadiazole–quinoline derivatives was good, the hybrids 120a and 120f having the best antimicrobial activity against B. cereus with an MIC of 17, respectively, 24 μg/mL, compared to standard drug ampicilin (MIC = 16 μg/mL).
Tahaab et al. [44] synthesized a series of oxadiazole–quinoline hybrids and studied their leishmanicidal potential. The reaction pathway to obtain the oxadiazole–quinoline hybrids 121a–r is depicted in Scheme 39.
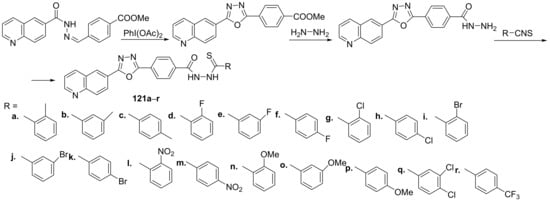
Scheme 39. Reaction pathway to obtain oxadiazole-quinoline hybrids 121a–r.
The synthesized oxadiazole-quinoline hybrids 121a–r were tested for their leishmanicidal activity against Leishmania major promastigote. Most of the synthesized hybrids have a good leishmanicidal activity, compound 121r was found to be the most active (IC50 = 0.10 μM) from the series, being 70 times more active than the standard drug (pentamidine, IC50 = 7 μM).
Irfan et al. [45] synthesized a series of triazole–quinoline hybrids and studied their antifungal properties. The reaction pathway involves a typical click cyclocondensation reaction of azide with a compound with a triple bond, when the desired triazole–quinoline hybrids 122a–c were obtained, Scheme 40.
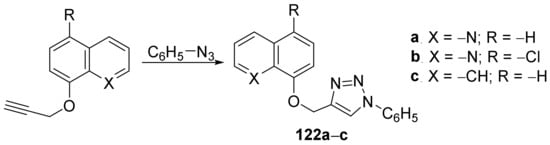
Scheme 40. Reaction pathway to obtain triazole-quinoline hybrids 122a–c.
The synthesized triazole–quinoline hybrids 122a–c were tested against fungus Candida albicans, both clinical isolates and laboratory strains [three FLC susceptible strains (C. albicans D27, C. albicans D31 and C. albicans D39) and one FLC resistant strain (C. albicans D15.9)]. The best antifungal activity was found for the hybrids 122a and 122b, having an MIC of 25 μg/mL for 122a and an MIC of 250 μg/mL for 122b, compared to control FLC (MIC >1 μg/mL)
Pandya et al. [46] synthesized a library of pyrazole–isoquinoline hybrids and studied their antimicrobial properties. The reaction pathway involves a palladium-catalyzed reaction of pyrazole derivatives with t-butyl-isocyanide, when the corresponding pyrazole–isoquinoline hybrids 123a–g, were obtained, Scheme 41.
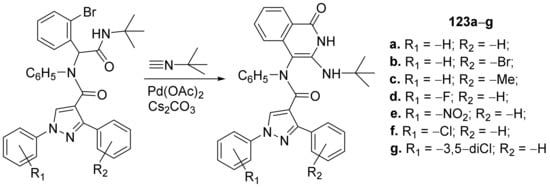
Scheme 41. Reaction pathway to obtain pyrazole-isoquinoline hybrids 123a–g.
The synthesized pyrazole–isoquinoline hybrids 123a–g were evaluated for their antimicrobial activity against different pathogenic strains: bacterial strains (Staphylococcus aureus, Escherichia coli, Enterococcus faecalis, Streptococcus pyogens and Vibrio cholera), fungal strains (Candida albicans, Candida glabrata, Candida krusei, Candida tropicalis and Candida parapsilosis) and tubercular strain (Mycobacterium tuberculosis). The antimicrobial activity of hybrids was very good, the hybrids 123e and 123g having the best antimicrobial activity, compared to standard drugs kanamycin and amphotericin B. Thus, the most active hybrids against S. aureus are 123e and 123g, having an MIC of 20 μM, respectively, 37 μM, compared to standard drug kanamycin (MIC of 31 μM). The most active hybrids against V. cholera are 123e and 123g, having an MIC of 41 μM, respectively, 90 μM, compared to the standard drug kanamycin (MIC of 62 μM). The hybrids 123e and 123g have the best antitubercular activity against M. tuberculosis with an MIC of 30 μg/mL, respectively, 32 μg/mL, compared to standard drugs rifampicin and isoniazide (MIC of 90 μg/mL).
Verma et al. [47] obtained a series of piperazine- and pyrimidine- isoquinoline hybrids and studied their antimicrobial properties. The piperazine-isoquinoline hybrids 126a–h were synthesized by condensation of the carboxylic acid intermediates 124a–d with appropriate aryl-piperazines, Scheme 38. The pyrimidine-isoquinoline hybrids 127a–h were synthesized in two steps: an O-alkylation of the carboxylic acid intermediates 124a–d (with ethylene dichloride), followed by an S-alkylation of the obtained compounds 125a–d (with thio-pyrimidine), Scheme 42.
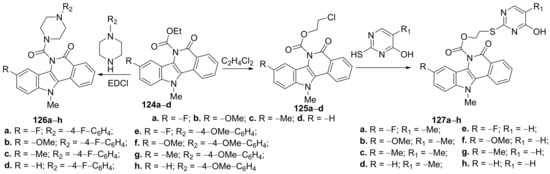
Scheme 42. Reaction pathway to obtain piperazine- and pyrimidine-isoquinoline hybrids 126a–h and 127a–h.
The synthesized piperazine- and pyrimidine-isoquinoline hybrids 124a–h and 125a–h were evaluated for their antibacterial and antifungal (Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Bacillus subtilis, Aspergillus niger, Aspergillus oryzae, Candida albicans and Pencillium chrysogenum), antioxidant, anticancer and antituberculosis (Mycobacterium tuberculosis) activities. The antibacterial assay indicates that three hybrids, namely 124a, 125a and 126e have the best activity against E. coli (with an MIC in the range of 1–3 μg/mL) and K. pneumoniae (with an MIC in the range of 1.5–3 μg/mL), compared with the standard drug ciprofloxacin (MIC = 1.5 μg/mL). The hybrids 125a, 126a and 127a also have excellentactivity against S. aureus (with an MIC in the range of 1–3 μg/mL) and B. subtilis (with an MIC in the range of 1.5–3 μg/mL), compared with the standard drug ciprofloxacin (MIC = 1.5 μg/mL, respectively, MIC = 3 μg/mL). The hybrids 125a, 126a and 127a have excellent activity against fungus A. niger, C. albicans, A. oryzae, and P. chrysogenum (with an MIC of 1.5 μg/mL), compared with the standard drug fluconazole (MIC = 1.5 μg/mL for A. niger and C. albicans, respectively, MIC = 3 μg/mL for A. oryzae, and P. chrysogenum). The hybrids 127b and 127e have the best activity against M. tuberculosis (MIC 1.0 mg/mL), compared with the standard drug rifampicin (MIC = 0.1mg/mL. The antioxidant and anticancer activity proved to be modest.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14102026
References
- WHO. Global Action Plan on Antimicrobial Resistance: WHO. 2016. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 12 April 2022).
- Bansal, Y.; Silakari, O. Multifunctional compounds: Smart molecules for multifactorial diseases. Eur. J. Med. Chem. 2014, 76, 31–42.
- Bremner, J.B.; Ambrus, J.I.; Samosorn, S. Dual action-based approaches to antibacterial agents. Curr. Med. Chem. 2007, 14, 1459–1477.
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973.
- Chourasiya, S.S.; Kathuria, D.; Wani, A.A.; Bharatam, P.V. Azines: Synthesis, structure, electronic structure and their applications. Org. Biomol. Chem. 2019, 17, 8486–8521.
- Amariucai-Mantu, D.; Antoci, V.; Sardaru, M.C.; Al Matarneh, C.M.; Mangalagiu, I.I.; Danac, R. Fused pyrrolo-pyridines and pyrrolo-(iso) quinoline as anticancer agents. Phys. Sci. Rev. 2022, 1.
- Amariucai-Mantu, D.; Mangalagiu, V.; Danac, R.; Mangalagiu, I.I. Microwave assisted reactions of azaheterocycles for medicinal chemistry applications. Molecules 2020, 25, 716.
- Luca, M.C.; Tura, V.; Mangalagiu, I.I. Considerations concerning design and mechanism of action of a new class of dual DNA intercalators. Med. Hypotheses 2010, 75, 627–629.
- Eryılmaz, S.; Turkcelikoglu, E.; Idil, O.; Inkaya, I.; Kozak, Z.; Mısır, E.; Gul, M. Derivatives of pyridine and thiazole hybrid: Synthesis, DFT, biological evaluation via antimicrobial and DNA cleavage activity. Bioorg. Chem. 2020, 95, 103476.
- Cinarli, M.; Ataol, C.Y.; Cinarli, E.; Idil, O. Synthesis, characterization, biological, X-ray diffraction analysis andcomputational chemistry studies of new 2-acetylpyridine derivativehydrazone and its Zn(II) complex. J. Mol. Struct. 2020, 1213, 128152.
- Trotsko, N.; Golus, J.; Kazimierczak, P.; Paneth, A.; Przekora, A.; Ginalska, G.; Wujec, M. Synthesis and antimycobacterial activity of thiazolidine-2,4-dione based derivatives with halogenbenzohydrazones and pyridinecarbohydrazones substituents. Eur. J. Med. Chem. 2020, 189, 112045.
- Sanad, S.M.H.; Ahmed, A.A.M.; Mekky, A.E.M. Efficient synthesis and molecular docking of novel antibacterial pyrimidines and their related fused heterocyclic derivatives. J. Heterocycl.Chem. 2020, 57, 590–605.
- Desai, N.C.; Bhatt, N.B.; Joshi, S.B.; Jadeja, K.A.; Khedkar, V.M. Synthesis, Antimicrobial Activity and 3D-QSAR Study of Hybrid Oxazine Clubbed Pyridine Scaffolds. ChemistrySelect 2019, 4, 7541–7550.
- Sribalan, R.; Banuppriya, G.; Kirubavathi, M.; Padmini, V. Synthesis, biological evaluation and in silico studies of tetrazole-heterocycle hybrids. J. Mol. Struct. 2019, 1175, 577–586.
- Kuthyala, S.; Shankar, M.K.; Nagaraja, G.K. Synthesis, Single-Crystal X-ray, Hirshfeld and Antimicrobial Evaluation of some New Imidazopyridine Nucleus Incorporated with Oxadiazole Scaffold. ChemistrySelect 2018, 3, 12894–12899.
- Ahirwar, J.; Ahirwar, D.; Lanjhiyana, S.; Jha, A.K.; Dewangan, D.; Badwaik, H. Synthesis, Characterization, Molecular Modeling, and Biological Evaluation of 1,2,4-Triazole-pyridine Hybrids as Potential Antimicrobial Agents. J. Heterocycl. Chem. 2018, 55, 2598–2609.
- Jaabil, G.; Ranganathan, R.; Ponnuswamy, A.; Suresh, P.; Shanmugaiah, V.; Ravikumar, C.; Murugavel, S.A. Green and Efficient Synthesis of Bioactive 1, 2, 3-Triazolyl-Pyridine/Cyanopyridine Hybrids via One-Pot Multicomponent Grinding Protocol. ChemistrySelect 2018, 3, 10388–10393.
- Flefel, E.M.; El-Sofany, W.I.; El-Shahat, M.; Naqvi, A.; Assirey, E. Synthesis, molecular docking and in vitro screening of some newly synthesized triazolopyridine, pyridotriazine and pyridine-pyrazole hybrid derivatives. Molecules 2018, 23, 2548.
- Amperayani, K.R.; Kumar, K.N.; Parimi, U.D. Synthesis and in vitro and in silico antimicrobial studies of novel piperine–pyridine analogs. Res. Chem. Intermed. 2018, 44, 3549–3564.
- Albayrak, F.; Çiçek, M.; Alkaya, D.; Kulu, I. Design, synthesis and biological evaluation of 8-aminoquinoline-1,2,3-triazole hybrid derivatives as potential antimicrobial agents. Med. Chem. Res. 2022, 31, 625–665.
- Hryhoriv, H.; Mariutsa, I.; Kovalenko, S.M.; Georgiyants, V.; Perekhoda, L.; Filimonova, N.; Geyderikh, O.; Sidorenko, L. The Search for New Antibacterial Agents among 1,2,3-Triazole Functionalized Ciprofloxacin and Norfloxacin Hybrids: Synthesis, Docking Studies, and Biological Activity Evaluation. Sci. Pharm. 2022, 90, 2.
- Hryhoriv, H.; Mariutsa, I.; Kovalenko, S.M.; Sidorenko, L.; Perekhoda, L.; Filimonova, N.; Geyderikh, O.; Georgiyants, V. Structural modification of ciprofloxacin and norfloxacin for searching new antibiotics to combat drug-resistant bacteria. Sci. Pharm. Sci. 2021, 5, 4–11.
- Drweesh, E.A.; Kucharova, V.; Volarevic, V.; Miloradovic, D.; Ilic, A.; Radojevic, I.D.; Rakovic, I.R.; Smolkova, R.; Vilkova, M.; Sabolov, D.; et al. Low-dimensional compounds containing bioactive ligands. Part XVII: Synthesis, structural, spectral and biological properties of hybrid organic-inorganic complexes based on 2− with derivatives of 8-hydroxyquinolinium. J. Inorg. Biochem. 2022, 228, 111697.
- Nehra, N.; Tittal, R.K.; Ghule, V.D. 1,2,3-Triazoles of 8-Hydroxyquinoline and HBT: Synthesis and Studies (DNA Binding, Antimicrobial, Molecular Docking, ADME, and DFT). ACS Omega 2021, 6, 27089–27100.
- Awolade, P.; Cele, N.; Kerru, N.; Singh, P. Synthesis, antimicrobial evaluation, and in silico studies of quinoline—1H-1,2,3-triazole molecular hybrids. Mol. Divers. 2021, 25, 2201–2218.
- Ammar, Y.A.; El-Hafez, S.M.A.A.; Hessein, S.A.; Ali, A.M.; Askar, A.A.; Ragab, A. One-pot strategy for thiazole tethered 7-ethoxy quinoline hybrids: Synthesis and potential antimicrobial agents as dihydrofolate reductase (DHFR) inhibitors with molecular docking study. J. Mol. Struct. 2021, 1242, 130748.
- Eissa, S.I.; Farrag, A.M.; Abbas, S.Y.; El Shehry, M.F.; Ragab, A.; Fayed, E.A.; Ammar, Y.A. Novel structural hybrids of quinoline and thiazole moieties: Synthesis and evaluation of antibacterial and antifungal activities with molecular modeling studies. Bioorg. Chem. 2021, 110, 104803.
- Lagdhir, M.; Pandya, C.; Pandya, A.; Vekariya, R.H.; Rajani, D.P. Design and synthesis of new quinoline hybrid derivatives and their antimicrobial, antimalarial and antitubercular activities. Indian J. Chem. Sect. B 2021, 60, 986–998.
- Desai, N.C.; Harsora, J.P.; Mehta, H.K. 2-Pyridone quinoline hybrids as potent antibacterial and antifungal agents. Indian J. Chem. Sect. B 2021, 60, 261–266.
- Vishnuvardhan, M.; Pradeep, M.; Gangadhar, T. An efficient microwave assisted synthesis and antimicrobial activity of novel p-Tolyloxyquinoline-Triazole hybrid derivatives. Chem. Data Collect. 2021, 31, 100612.
- Abdel-Rahman, I.M.; Mustafa, M.; Mohamed, S.A.; Yahia, R.; Abdel-Aziz, M.; Abuo-Rahma, G.; Hayallah, A.M. Novel Mannich bases of ciprofloxacin with improved physicochemical properties, antibacterial, anticancer activities and caspase-3 mediated apoptosis. Bioorg. Chem. 2021, 107, 104629.
- Mohammed, A.A.M.; Suaifan, G.A.R.Y.; Shehadeh, M.B.; Okechukwu, P.N. Design, synthesis and antimicrobial evaluation of novel glycosylated-fluoroquinolones derivatives. Eur. J. Med. Chem. 2020, 202, 112513.
- Shruthi, T.G.; Subramanian, S.; Eswaran, S. Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids. Heterocycl. Commun. 2020, 26, 137–147.
- Kaur, G.; Kaur, M.; Sharad, L.; Bansal, M. Theoretical molecular predictions and antimicrobial activities of newly synthesized molecular hybrids of norfloxacin and ciprofloxacin. J. Heterocycl. Chem. 2020, 57, 225–237.
- Insuasty, D.; Vidal, O.; Bernal, A.; Marquez, E.; Guzman, J.; Insuasty, B.; Quiroga, J.; Svetaz, L.; Zacchino, S.; Puerto, G.; et al. Antimicrobial activity of quinoline-based hydroxyimidazolium hybrids. Antibiotics 2019, 8, 239.
- Baartzes, N.; Stringer, T.; Seldon, R.; Warner, D.F.; Taylor, D.; Wittlin, S.; Chibale, K.; Smith, G.S. Bioisosteric ferrocenyl aminoquinoline-benzimidazole hybrids: Antimicrobial evaluation and mechanistic insights. Eur. J. Med. Chem. 2019, 180, 121–133.
- Fedorowicz, J.; Sączewski, J.; Konopacka, A.; Waleron, K.; Lejnowski, D.; Ciura, K.; Toma, T.; Skok, Z.; Savijoki, K.; Morawska, M.; et al. Synthesis and biological evaluation of hybrid quinolone-based quaternary ammonium antibacterial agents. Eur. J. Med. Chem. 2019, 179, 576–590.
- Borazjani, N.; Jarrahpour, A.; Rad, J.A.; Mohkam, M.; Behzadi, M.; Ghasemi, Y.; Mirzaeinia, S.; Karbalaei-Heidari, H.R.; Ghanbari, M.M.; Batta, G.; et al. Design, synthesis and biological evaluation of some novel diastereoselective β-lactams bearing 2-mercaptobenzothiazole and benzoquinoline. Med. Chem. Res. 2019, 28, 329–339.
- Berry, L.; Domalaon, R.; Brizuela, M.; Zhanel, G.G.; Schweizer, F. Polybasic peptide-levofloxacin conjugates potentiate fluoroquinolones and other classes of antibiotics against multidrug-resistant Gram-negative bacteria. MedChemComm 2019, 10, 517–527.
- Mermer, M.; Faiz, O.; Demirbas, A.; Demirbas, N.; Alagumuthu, M.; Arumugam, V. Piperazine-azole-fluoroquinolone hybrids: Conventional and microwave irradiated synthesis, biological activity screening and molecular docking studies. Bioorg. Chem. 2019, 85, 308–318.
- Guo, Y.; Xu, T.; Bao, C.; Liu, Z.; Fan, J.; Yang, R.; Qin, S. Design and synthesis of new norfloxacin-1,3,4-oxadiazole hybrids as antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Pharm. Sci. 2019, 136, 104966.
- Wang, Y.N.; Bheemanaboina, R.R.Y.; Gao, W.W.; Cang, J.; Cai, G.X.; Zhou, C.H. Discovery of Benzimidazole–Quinolone Hybrids as New Cleaving Agents toward Drug-Resistant Pseudomonas aeruginosa DNA. ChemMedChem 2018, 13, 1004–1017.
- Bharadwaj, S.S.; Poojary, B.; Kumar, M.S.; Byrappa, K.; Nagananda, K.S.; Chaitanya, A.K.; Zaveri, K.; Yarla, N.S.; Shiralgi, Y.; Kudva, A.K.; et al. Design, synthesis and pharmacological studies of some new quinoline Schiff bases and 2,5-(disubstituted-)-oxadiazoles. New J. Chem. 2017, 41, 8568–8585.
- Tahaab, M.; Ismailab, N.H.; Alic, M.; Rashidc, U.; Imranab, S.; Uddind, N.; Khane, K.M. Molecular hybridization conceded exceptionally potent quinolinyl-oxadiazole hybrids through phenyl linked thiosemicarbazide antileishmanial scaffolds: In silico validation and SAR studies. Bioorg. Chem. 2017, 71, 192–200.
- Irfan, M.; Alam, S.; Manzoor, N.; Abid, M. Effect of quinoline based 1,2,3-triazole and its structural analogues on growth and virulence attributes of Candida albicans. PLoS ONE 2017, 12, e0175710.
- Pandya, K.M.; Battula, S.; Naik, P.J. Pd-catalyzed post-Ugi intramolecular cyclization to the synthesis of isoquinolone-pyrazole hybrid pharmacophores & discover their antimicrobial and DFT studies. Tetrahedron Lett. 2021, 81, 153353.
- Verma, V.A.; Saundane, A.R.; Meti, R.S.; Vennapu, D.R. Synthesis of novel indoloisoquinoline derivatives bearing pyrimidine, piperazine rings and their biological evaluation and docking studies against COVID-19 virus main protease. J. Mol. Struct. 2021, 1229, 129829.
This entry is offline, you can click here to edit this entry!
