Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell & Tissue Engineering
Polymers are sustainable and renewable materials that are in high demand due to their excellent properties. Natural and synthetic polymers with high flexibility, good biocompatibility, good degradation rate, and stiffness are widely used for various applications, such as tissue engineering, drug delivery, and microfluidic chip fabrication. Indeed, the advances in microfluidic technology allow the fabrication of polymeric matrix to construct microfluidic scaffolds for tissue engineering and to set up a well-controlled microenvironment for manipulating fluids and particles.
- polymers
- microfluidics
- lab-on-chip
- biomedical engineering
1. Polymers Used in Microfluidic Devices
A single polymer unit may be composed of hundreds or millions of monomers. They have one of the four basic polymer structures: linear, branched, cross-linked, or networked (Figure 2). The two types of polymers are natural and synthetic. Natural polymers can be extracted from biological systems such as plants, algae, microorganisms, and animals, which have a similar ECM structure to native tissues. Synthetic polymers are similar to natural polymers, but they are much cheaper, can be produced at large scale, and have long shelf life compared with natural polymers. As such, generally, they present good cellular attachment, which improves the cellular behaviors and prevents immunological reactions [1,14].
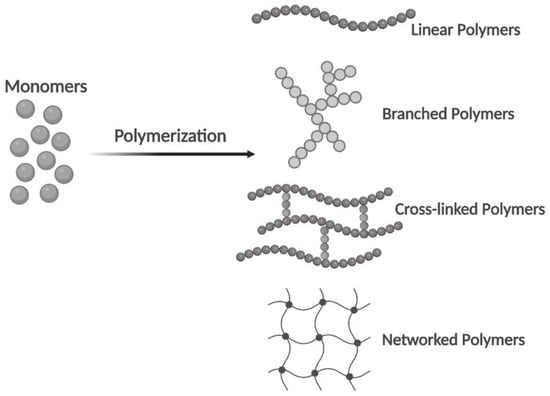
Figure 2. From monomers to polymers. Linear, branched, cross-linked, and networked structures in polymers (Created with Biorender.com, accessed on 12 October 2022).
Choosing the right material is the first and most critical step in designing a successful microfluidic device. A wide range of constraints and requirements dictates the selection of the material for a specific component. The design of the device, the compatibility of the material with the chemicals, as well as the applied temperature and pressure are crucial considerations in material selection. Additionally, the final application of the device is an essential consideration. For example, devices intended for in vivo applications in tissue engineering must be nontoxic, exhibit a slow and predictable degradation rate, have nontoxic and safe degradation products, and potentially capable of mimicking certain physical and chemical properties of the native ECM or of supporting other agents that play such roles. The architecture of the device can also influence the choice of materials. For example, in devices that contain microfluidic systems, the materials have to be mechanically robust but have a controlled degree of flexibility [15,16]. The material also has to be compatible with microfabrication techniques, easily processed in mild conditions, and cheap to manufacture, among others [17]. Polymers are classified into two major groups: biodegradable and biostable polymers. These two types of polymers are commonly used as scaffolds or bioactive coatings in biomedical applications [8,15]. The next section focuses on these two classes of polymers for the manufacture of microfluidic devices and their biomedical applications.
2. Biodegradable Polymers
Sustainable polymers from various renewable resources can be directly obtained from biomass (proteins and polysaccharides), or through chemical modifications of natural polymers [18]. However, there are many sources of natural and synthetic biodegradable polymers. Natural polymers are derived from natural raw materials and available in large quantities while synthetic polymers are synthesized by the chemical polymerization of bio-monomers.
2.1. Natural Biodegradable Polymers
The use of natural biopolymers in microfluidics provides many advantages, such as surface chemistry biocompatibility and having the same mechanical properties of the native proteins of interest [16,19].
Natural polymers, such as chitosan, alginate, and gelatin, are also biologically derived and biodegradable polymers. They are used in the manufacturing of biodevices that are intended to interact with the biological systems of the human body. The crosslinking ability of these natural polymers, which is induced by physical and chemical stimuli, makes them ideal for the preparation of microgels for microfluidic devices. The two natural biopolymers, alginate and gelatin, were used as substrates to make two types of hydrogel-based microfluidics. Subsequently, the fabricated hydrogel microchannels can be used as platforms to provide 3D cell culture environments for mammalian cells: fibroblasts and vascular endothelial cells. The developed enclosed microchannel models are simple and reproducible and do not require complicated operations [16,19].
One class of natural biomaterials that is a good candidate for microfluidic devices is silk fibroin (SF) [20]. SF protein, originally found in the silkworm Bombyx mori, is a high-molecular-weight protein that primarily consists of hydrophobic residues. This protein is approved by Food and Drug Administration (FDA) for many medical applications, such as drug delivery and tissue engineering. SF can be easily processed to form hydrogels, films, and nanofiber mats under mild conditions [21]. In recent years, SF has also been used to fabricate microfluidic devices due to its excellent biocompatibility, robust mechanical properties, and slow proteolytic degradation rate [16,20]. The solubility and mechanical properties of SF materials are linked to its secondary structure. Whereas self-assembled β-sheet structures are responsible for the mechanical stability and water insolubility of SF, the amorphous regions, including random coil, α helix, and β turn structures, contribute to the elasticity and solubility of the biomaterial. Thus, SF-based microfluidic fabrication strategies allow the rapid and scalable production of devices without the need for harsh processing conditions that require cytotoxic reagents. Mao et al. used SF and chitosan to construct porous SF–CS scaffolds with predefined microfluidic channels. The generated model showed structural properties suitable for seeding and growth of hepatic cells. Mass transport and uniform cell distribution within the 3D scaffold were successfully achieved [19].
In addition to all the above-mentioned advantages of natural polymers, the inclusion of natural ECM proteins into microfluidic devices allows the reproduction native cell–biomaterial interactions in vitro [22]. The use of ECM proteins is crucial in controlling cell function overall via other physicochemical mechanisms such as specific cell binding domain sequences. Proteins, such as fibronectin, vitronectin, and collagen I, contain the amino acid sequence of arginine–glycine–aspartic acid (RGD), which supports the adhesion of cells and to control stem cell differentiation. For example, Arik et al. reported the fabrication of a collagen-I-based membrane incorporated in an organ-on-chip device [23]. The membrane demonstrated permeability, as well as the adhesion of both endothelial and epithelial cells. Moreover, they characterized the degradation and remodeling of the basement membrane by a protease. Natural proteins offer an environment that more closely mimics that of the body and more realistically mimics the cell–ECM interactions, which are crucial for tissue engineering. However, these biomaterials have a complex structural composition that prevents complete control over their composition and other factors, such as molecular weight, immune response, degradation, and mechanical properties. As an alternative, scientists have focused on the development and use of synthetic polymers, which have more tunable properties [22].
2.2. Synthetic Biodegradable Polymers
Synthetic polymers were proposed as ideal candidates for the fabrication of biodegradable microstructures, including microfluidic biomaterials [16,24]. Poly(glycolic acid) (PGA), poly(lactic acid) (PLA), and their copolymer poly(lactic acid-co-glycolic acid) (PLGA), belong to the linear aliphatic polyesters family [25] (Figure 3). This polymer family is one of the most widely used in tissue engineering and drug delivery [25,26]. These polymers have several advantages, such as low cost, ease of processing, and well-characterized biological behavior. These polymers (PLA, PGA, and PLGAs) are among the few synthetic polymers approved by the U.S. FDA for certain human clinical applications [26]. These polymers degrade through the hydrolysis of the ester bonds [27]. Although PGA and PLA belong to the same family, they also display distinct properties. For instance, because of its very hydrophilic nature, PGA rapidly degrades in aqueous solutions. However, PGA and PLA show the same behavior in vivo: they lose mechanical integrity in a period between two and four weeks [28]. Conversely, PLA contains a methyl group, which renders the chains more hydrophobic and hence reduces the affinity to water, and displays a slower hydrolysis rate (months to years) [28]. This class of biodegradable polymers is suitable for microfluidics because of the wide range of tunable properties [27]. They can be modulated by adjusting the lactide-to-glycolide ratio.
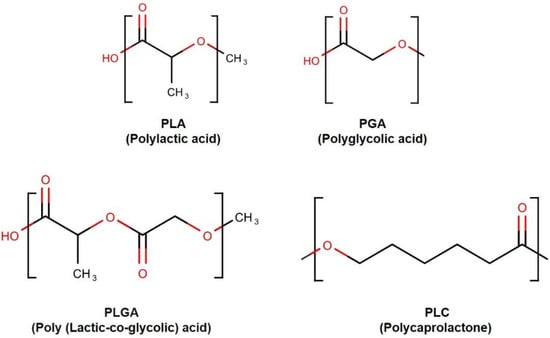
Figure 3. Synthetic bio-degradable polymer structures.
The physical properties of the copolymer PLGA are defined by the properties of both pure PGA and PLA [25]. The presence of PLA makes it more hydrophobic than PGA. Hence, lactide-rich PLGA copolymers are less hydrophilic and more slowly degrade. Additionally, PLA exhibits relatively a high glass transition temperature (Tg = 50–80 °C) and melting point (Tm = 173–178 °C). PLGA blends of various copolymer ratios exhibit a reduced phase transition temperature (Tg, PLGA75/25 = 54 °C) and melting point (Tm, PLGA75/25 = 80 °C) [29]. Poly(caprolactone) (PCL) is another example of an aliphatic polyester used in microfluidics. PCL demonstrates advantageous properties for replica molding strategies, such as a low melting point (Tm = 57 °C) and low glass-transition temperature (Tg = −62 °C) [30]. PCL can be degraded by micro-organisms as well as by the hydrolysis of its ester linkage in physiological conditions [31]. However, PCL materials have a substantially slower biodegradation rate than PLA and PGA, making it suitable for the use in long-term implantable systems. Biodegradable cell-support scaffolds play an important role in the growth of engineered tissue and the delivery of biologically active agents. Therefore, the concept of biodegradable microfluidic devices formed by various biodegradable polymers has attracted considerable research attention. For example, microstructured PLGA films were used to construct a high-resolution and high-precision 3D device. The developed device allows diffusion distance reduction in cell-seeded scaffolds with convective transport [32]. PLA microchannels have been widely generated by 3D printing. Kadimisetty et al. developed a microfluidic immunoarray using PLA and a 3D printer. The fabricated device was low cost and could sensitively detect prostate cancer biomarker proteins [33].
Poly(1,3-diamino-2-hydroxypropane-co-polyol sebacate) (APS) is another biodegradable elastomeric polymer used to construct microfluidic scaffolds. The simple microchannel network design exhibited a very low degradation rate while retaining the elastomeric properties required for tissue scaffold applications [24].
3. Biostable Polymers
PDMS is a mineral–organic polymer structurally composed of silane-oxygen backbones covered with alkyl groups. Depending on the size of the monomer chain, non-cross-linked PDMS may be almost liquid (low amount of n monomer) or semi-solid (high amount of n monomer) [34]. The high level of viscoelasticity displayed by the polymer chain is due to the siloxane bonds in the polymer structure. After cross-linking with a curing agent, PDMS becomes a hydrophobic elastomer [34]. One of the main reasons for the success of PDMS in microfluidics is the ease of PDMS device fabrication, which also allows mass production. Among many other methods, PDMS microchips can be fabricated through microscale molding processes [35]. For example, a silicon wafer with patterns can be used as a mold master. Prepolymer PDMS is poured into the mold master. Then, cured PDMS is peeled off from the master to be pasted on a flat plate, i.e., PMMA, glass, etc. [34]. The flat support should be drilled in advance to provide access ports for the introduction of reagents and samples. PDMS can precisely replicate structures down to the submicron size [36]. Due to the favorable optical properties of PDMS (almost no absorbance in the visible wavelength range), fluorescent dyes are widely used for the detection and quantification of molecules in most biochemical analyses. In addition, PDMS is transparent, biocompatible, nontoxic, and displays high gas permittivity, so has been traditionally used as a biomaterial in catheters, insulation for pacemakers, and ear and nose implants [10].
The combination of its elastic properties, easy processability, and the other properties mentioned above make PDMS an ideal candidate for use in microfluidic devices for biomedical and cell applications.
Many studies have been performed to further examine the compatibility of PDMS with both microfluidic technology and biomedical applications [37]. In terms of microfluidic technology, the effects of the structure and surface of PDMS in widely used microfluidics methods, such as spin coating and chemical immersion, on different liquid chemicals have been studied. Successful spin-coating of PDMS depends on the crosslinking ratio; increased amounts of crosslinker agent in the formulation decrease film thickness. Additionally, whereas chemical immersion (solvents such as alcohol, toluene, acetone, etc.) does not result in major changes in the surface hydrophilicity of PDMS, macrotexture distortion and destructions are observed with strong acids (hydrofluoric, nitric, sulfuric, and hydrofluoric acids) and bases (potassium hydroxide). For biomedical applications, the effect of oxygen plasma and sterilization and the exposure to tissue culture media was also explored. Oxygen plasma exposure increases PDMS surface hydrophilicity, whereas a following exposure to air leads to hydrophobic recovery. UV and alcohol sterilization do not affect the PDMS surface microtexture, element concentration, hydrophilicity, or mechanical properties. Finally, immersion in tissue culture media increases the surface concentration of oxygen relative to silicon [38].
Despite all these advantages, the use of PDMS is limited due to challenges encountered in microfluidics. For example, incomplete curing of PDMS leaves uncrosslinked oligomers within the material, which can leach out and contaminate the culture medium. Other problems, such as incompatibility with some organic solvents, water evaporation, channel deformation, and adsorption of biomolecules onto channel walls, present severe limitations to the use of PDMS for microfluidics applications [39].
Thermoplastics
Thermoplastics are plastic polymer materials that have emerged as a commercially viable material. Their use has recently increased, being widely applied to fabricate microfluidics platforms for biomedical applications. The most commonly used thermoplastics are PMMA, polycarbonate (PC), polystyrene (PS), polyvinyl chloride (PVC), Cyclo-olefin-copolymer (COC), and Cyclo-olefinpolymer (COP) [39,40] (Figure 4).
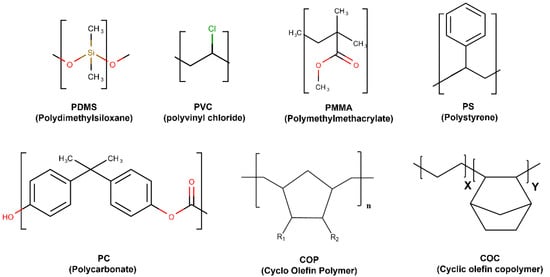
Figure 4. Most used thermostable polymers structures for microfluidic chips.
Because of their linear structure, their thermoplastic rigidity resists temperature and pressure changes. The properties of the most common thermoplastics used for chips fabrication are summarized in Table 1. Thermoplastic-based materials have good physical and chemical characteristics, such as high chemical and mechanical stability; low water-absorption capacity; acid/base resistivity; and are suitable for mass production at low cost. In term of fabrication, thermoplastics can be softened after exposure to heat at their transition temperature (Tg), making them processable around this temperature. During cooling, the softened polymer hardens, and it takes the shape of the container or mold, without any chemical change. They can be reshaped multiple times by reheating, which is important for the molding and microfluidics fabrication process [41].
Table 1. Properties of the most used biocompatible thermoplastics in the microfluidic field.
| Thermoplastics | Young’s Modulus (Gpa) | Tg (°C) |
Tm (°C) | Solubility Parameter δ (MPa)1/2 |
Water Adsorption (%) |
O2 Permeability (×10−13 cm3 .cm cm −2 s−1 Pa−1) |
Transparency | Auto-fluorescence | Study |
|---|---|---|---|---|---|---|---|---|---|
| Polymethylmethacrylate (PMMA) |
2.4–3.4 | 105 | 250–260 | 20.1 | 0.1–0.4 | 0.1 | Transparent | Low | [42] |
| Polyethylene terephthalate (PET) |
2–2.7 | 70 | 255 | 20.5 | 0.16 | 0.03 | Transparent | Medium | [43] |
| Polypropylene (PP) |
1.5–2 | −20 | 160 | 16.3 | 0.01–0.1 | 1.7 | Both opaque and transparent | Medium | [41,44] |
| Polystyrene (PS) |
3–3.5 | 95 | 240 | 18.7 | 0.02–0.15 | 2 | Transparent | High | [45] |
| Polycarbonate (PC) |
2.6 | 145 | 260–270 | 19.4 | 0.23 | 1 | Transparent | High | [41] |
| Polyvinyl chloride (PVC) |
2.4–4.1 | 80 | 100–260 | 19.4 | 0.04–0.4 | 0.04 | Transparent | High | [46] |
| Polyamide (Nylon) |
2.5 | 47–60 | 190–350 | 28 | 1.6–1.9 | 0.03 | Transparent | High | [47] |
| Polytetrafluoroethylene (PTFE) |
0.4 | 115 | 326 | 12.6 | 0.005–0.01 | 3 | Transparent | High | [48] |
| Polyetheretherketone (PEEK) | 4–24 | 143 | 343 | 21.9 | 0.1–0.5 | 0.1 | Opaque | N/A | [49] |
One of the first properties to consider in cell biology is biocompatibility. According to Table 1, most of the thermoplastics are biocompatible. However, for long-term applications, some of the materials can be problematic. For example, polycarbonates can be experience surface erosion during in vivo applications. In addition, bisphenol A (BPA), which is hazardous in food contact situations, might be released during hydrolysis.
PVC can release toxic gases during manufacturing, and nylon is a heat-sensitive material. Resistance to solvents is also a main criterion that must be considered for microdevice fabrication and biomedical applications (sterility). PS is widely used in molecular and cell biology studies due to its biocompatibility and its high resistivity to alcohols, polar solvents, and alkalis [50]. PMMA is affected by ethanol, isopropyl alcohol, acetone, and other important solvents used in microfabrication and sterilization [51]. When working with cell cultures, low water absorption is beneficial because the cells consume more oxygen from water, which can be limited by the absorption of water onto the polymer surface.
The optical properties of the selected material (e.g., transparency and autofluorescence) are crucial. Consequently, PMMA, polyethylene terephthalate (PET), and polypropylene (PP) are less suitable for applications that require further reactions inside the microfluidic devices under a microscope. Additionally, PC displays high autofluorescence, so PC is difficult to use when working with fluorescently labelled cells or materials. In contrast, PS has high transparency, and the surface of PS is suitable for long-term cell studies [41]. Table 2 highlights some studies that used polymers as a chassis or to functionalize sensing surface.
Table 2. Some studies using polymers for microfluidics devices for various biological applications.
| Polymer | Cell Type | Application | Study |
|---|---|---|---|
| Polydimethylsiloxane (PDMS) | Alveolar epithelial cells, Macrophages, Mycobacterium tuberculosis | Rapid and uncontrolled bacterial growth in the mammalian cells can cause a surfactant deficiency in the lung-on-chip infection model. | [52] |
| PDMS, Carboxymethylated cellulose nanofibrils (CNF) |
HCT 116 colon cancer cell | The functionalized chip was able to capture the cancer cells from the whole blood with >97% efficiency which may use as rapid diagnostic tool. | [53] |
| PDMS, Dimethylallylamine (DMAA) |
Escherichia coli | The encapsulated bacteria with a membrane with a selective permeability of tetracycline cultured on the PDMS composition and functionalized with DMAA inhibit the bacterial growth which can be used as a diagnostic tool to evaluate the bacterial resistance. | [54] |
| PDMS microchannel layer and PDMS membrane | Human mesenchymal stem cells (hMSCs) | The two layer-microfluidic chips with three different stretching modes (uniaxial, radial, and gradient) showed different cell responses which may enhance the study of cells on biomaterials under various stretching stimuli. | [55] |
| Combination between PDMS and polymer substrate using a PrimeCoat-Epoxy adhesive layer by selective stamp bonding | Human lung epithelial cells | The cells cultured inside the device showed a similar viability comparing to the conventional cell culture technique. | [56] |
| Rapidly Integrated Debubbler (RID) from PMMA | human umbilical vein endothelial cells | The RID module showed a potential method to prevent the bubble entry into the microfluidics which may lead to device delamination and cell damage. | [57] |
| PDMS champers separated by thin layer of polyester (PE) membrane | Primary human small airway epithelial cells |
The microfluidics airway system showed a highly controllable and readily accessible physiologic pulmonary environments tailored for lung epithelial cells. | [58] |
| Combination of PDMS hydrophilic surface treatment and vacuum filling system equipped with bubble trap. | Mouse pancreatic islets | The system showed normal cell viability and morphology, normal insulin secretion, and normal intracellular calcium signaling. | [59] |
| PDMS | Endothelial cells | The actin filaments alignments directions of the cells cultured in microfluidics channels was significantly higher compering to the cells cultured in the static condition. | [60] |
| PDMS-glass | Human umbilical vein endothelial cells (VECs) | The synergistic effect of wall shear stress (WSS) and adenosinetriphosphate (ATP) signals played a vital role in the VEC Ca2+ signal transduction on the microfluidic device. | [61] |
| Photopolymer and chitosan | Hepatic oval cells (HOCs) | Electrochemical sensor is developed to rare cancer cells. Photopolymer is used to construct a 3D-printed continuous flow system and a chitosan film is served as a scaffold for the immobilization of anti-OV6-antibodies. | [62] |
This entry is adapted from the peer-reviewed paper 10.3390/polym14235132
This entry is offline, you can click here to edit this entry!
