Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Peptidomimetics are synthetically altered peptides with adjusted molecular properties for specific biological or therapeutic applications and have been an important class of drug molecules due to their potential features, high potency, and low toxicity since the term was created first in the late 1970s.
- opioid receptors
- analgesic drugs
- bioavailability
- peptidomimetic
1. Opioid Receptors and Natural Opioid Peptides
Opioid receptors which belong to G-protein coupled receptors are known to be involved in pain modulation, numerous physiological functions, and behavioral effects and are characterized in three subtypes, mu- (MOR), delta- (DOR), and kappa-opioid receptor (KOR) with overall 60–65% high structural homology [1]. The extracellular region has much lower homology, and the differences in the region are responsible for the subtype-selectivity of endogenous opioid peptides [2]. There are three main families of the endogenous opioid peptides, END, ENK, and DYN, which are derived from three different precursor proteins, pro-ENK, pro-DYN, and pro-opiomelanocortin, and prefer to bind at the MOR, DOR, and KOR, respectively, with low selectivity but strong analgesic effects in vivo with milder side effects, unlike morphine [1]. Regardless of the receptor selectivity, all of the endogenous opioid peptides share the same N-terminal tetrapeptide sequence (YGGF) that acts as the message part for the receptor, while their C-terminal acts as the address part for selectivity (Table 1).
Table 1. Natural opioid peptides and their selectivities for the opioid receptors.
| Peptides | Structure | Selectivity |
|---|---|---|
| β-Endorphin (END) | YGGFMTSEKSQTPLVTLFKNAIIKNAYKKGE | MOR > DOR |
| Enkephalins (ENKs) | YGGFL | DOR > MOR |
| YGGFM | DOR > MOR | |
| YGGFMRF | MOR > DOR > KOR | |
| YGGFMRGL | MOR > DOR > KOR | |
| Dynorphin (DYN) A | YGGFLRRIRPKLKWDNQ | KOR > MOR > DOR |
| DYN B | YGGFLRRQFKVVT | KOR > MOR > DOR |
| Endomorphin (EM)-1 | YPWF-NH2 | MOR |
| EM-2 | YPFF-NH2 | MOR |
| Dermorphines (DERs) | YaFGYPS-NH2 | MOR |
| YaFGYPK | MOR | |
| YaFWYPN | MOR | |
| Deltorphine (DLT) A | YmFHLMD-NH2 | DOR |
| DLT-1 | YaFDVVG-NH2 | DOR |
| DLT-2 | YaFEVVG-NH2 | DOR |
2. Peptidomimetics for Opioid Receptors
2.1. Pharmacological Aspect
2.1.1. Improving Bioactivity, Specificity, and Selectivity for the Opioid Receptors
Structural homology and the natural flexibility of endogenous linear opioid peptides limit their uses for a specific subtype receptor because of the possible interactions with more than one subtype receptors and drastic SAR results caused by subtle structural change. For this reason, selectivity, mostly between MOR and DOR, has been a major problem for the linear opioid peptides, and tremendous efforts have been made to reduce their structural flexibility and thereby enhance the subtype selectivity and potency. Incorporation of a constrained amino acid, configurational change from L to D, and cyclization of a backbone have been the most useful tool to achieve high selectivity and specificity. Figure 1 displays highly selective opioid peptidomimetics derived from ENK and DYN A for respective subtypes with high potency. DAMGO is a potent and selective MOR agonist to be rendered by introducing an N-methyl group into ENK to reduce rotational freedom around the peptide bond (φ/ψ constraint) [3]. Its crystal structure bound to the MOR showed a very similar pose in the binding pocket to those of other small molecule morphinans [4]. DPDPE is the most successful example of cyclization through a disulfide bond that constrains the ENK to an active conformation state for the DOR and has been used the most for scientific purposes [5][6]. It is noteworthy that both selective molecules have originated from less selective ENK. [DAla3]-DYN A-(1-11)-NH2 is a highly selective KOR agonist with a similar affinity to DYN A-(1-11)-NH2 showing how simple substitution can affect receptor selectivity drastically [7].
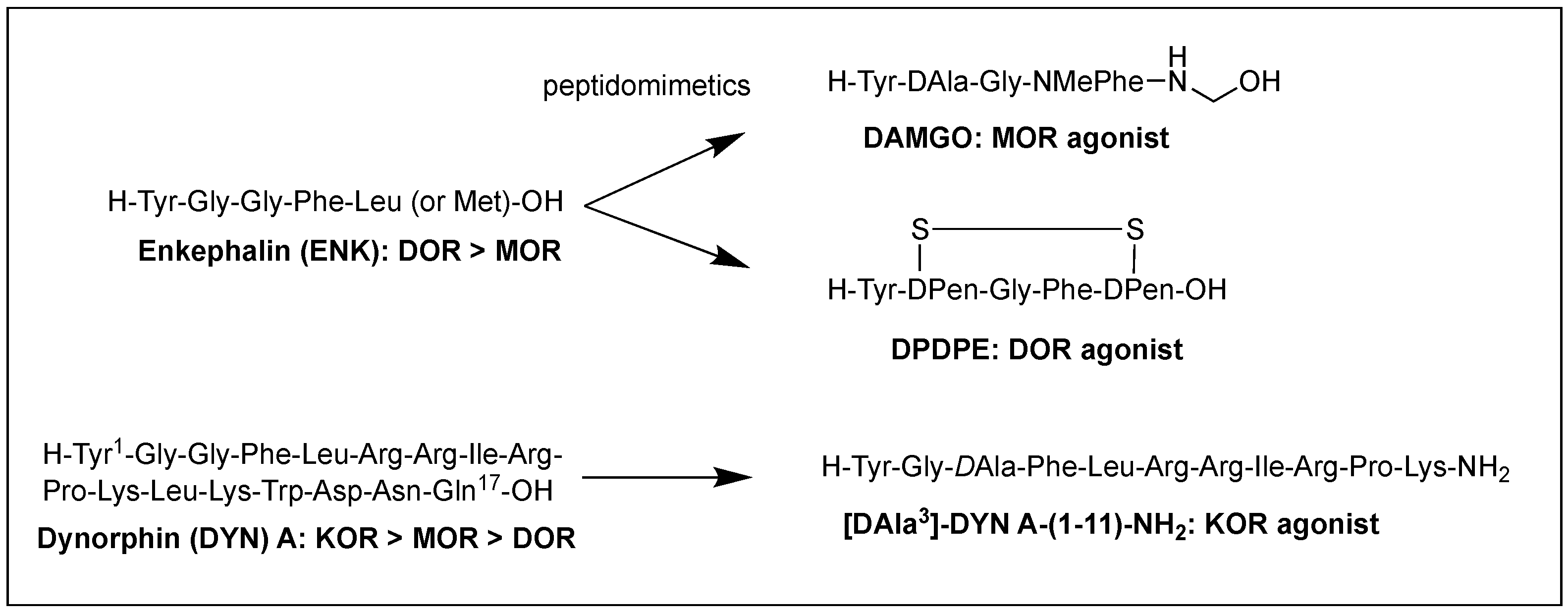
Figure 1. Subtype selective opioid peptidomimetics developed from endogenous opioid peptides.
2.1.2. Transforming Bioactivity from Agonism to Antagonism
While opioid agonists are used for pain relief, antagonists are also substantial in preventing or reversing the opioid agonist effects and intoxication as well as in pharmacological studies to investigate any endogenous opioid regulation. However, there is no opioid antagonist that naturally occurs, and currently available ones have been developed from opioid agonists by structural modifications. In general, agonist versus antagonist behavior of opioid peptides depends on very subtle structural differences, as shown in many cases [1][8][9].
The most effective and generalized structural modification for the conversion into an antagonist is the deletion of an Nα-amino group or its replacement with the other groups such as allyl and hydroxybenzyl [8][10][11]. These modifications similarly affect the three subtypes receptors. Substitution of Tyr1 with 3-(2,6-dimethyl-4-hydroxyphenyl)propanoic acid (Dhp) or (2S)-2-methyl-3-(2,6-dimethyl-4-hydroxyphenyl)propanoic acid (Mdp) converted opioid agonist peptides to corresponding antagonist peptides with high potency and selectivity [12]. The substitution of position 2 with 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) in ENK and DER analogs converted their respective agonist activities at DOR and MOR to DOR selective antagonists, and NMR analysis indicated a 90-degree arrangement of the two aromatic rings in the cis-Tyr-L-Tic moiety responsible for the conversion [13]. It was also shown that modifications of EM-2 and morphiceptin (YPFP-NH2) at position 3 with DPhe or D-2-Nal converted the MOR agonist activity to the antagonist activity [9]. Details are discussed in the later part, 3.2. Structural aspects.
2.1.3. Optimizing Drug-like Characteristics including Metabolic Stability, BBB Permeability, and Oral Bioavailability
Clinical application of opioid peptides has been limited due to poor metabolic stability in the body and low ability to cross the BBB [14]. The ability to cross the BBB and reach the CNS to interact with opioid receptors for analgesic effects relies on physicochemical properties including size, lipophilicity, enzymatic stability, hydrogen bonding potential, and other structural features [15]. Physicochemical properties of opioid peptides have been far from optimal and have restricted their oral bioavailability. The nonpeptidic nature of peptidomimetics has the potential to overcome the detrimental therapeutic characteristics and prolong and enhance biological activities at the target site [14][16]. Therefore, various peptidomimetic approaches have been devised to develop molecules with optimized CNS activity, although there are still no definitive alternatives to using the classical analgesics for pain treatment [15][17].
The difficulty of eliciting analgesia with three endogenous opioid peptides is mainly due to the rapid cleavage of the Tyr1-Gly2 bond, and simple modification of Gly2 with DAla2 in ENK resulted in long-lasting analgesic effects by avoiding the critical degradation [17][18]. Replacing a peptide bond susceptible to peptidases with a dipeptide isostere such as olefin, ester, and triazole has also been a useful tool to increase peptide stability as well as conjugating with antibodies [17][19][20]. A recent study showed that β-Ala at the N-terminus of an ENK-like tetrapeptide amide resulted in longer analgesic effects owing to less accessibility of endopeptidases [21]. Another recent study showed that Nα-guanidinyl group at the N-terminus, which mimics the ionic state of the parent peptide, significantly improved the stability and lipophilicity as well as affinity and potency, when combined with C-terminal tetrazolate and DAla in guanidyl-Tyr-DAla-Gly-Phe-Leu-tetrazole (Figure 2) [22].
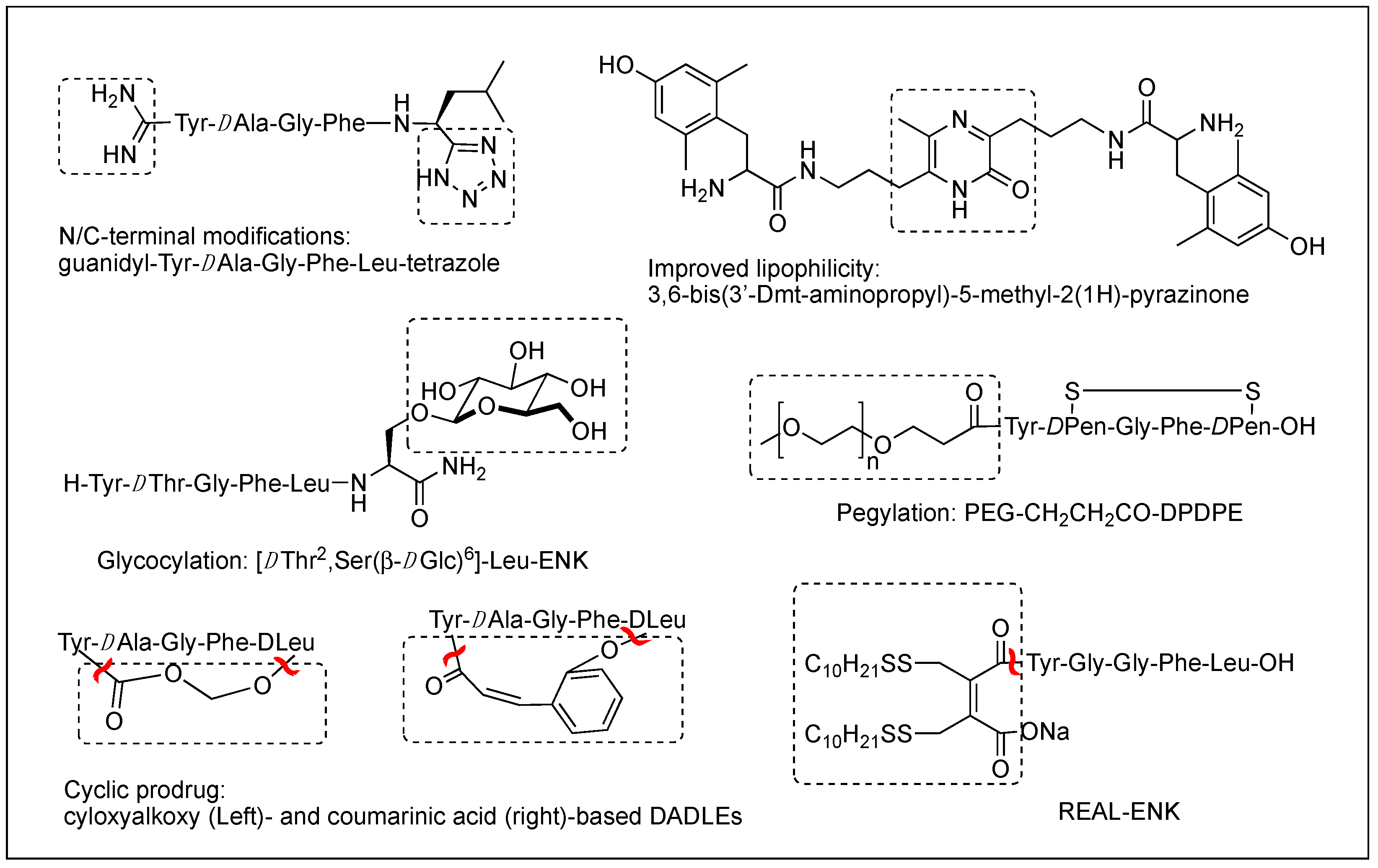
Figure 2. Various modifications to optimize metabolic stability, BBB permeability, and oral bioavailability.
Although metabolic stability has been improved by various structural modifications, the efforts to improve the drug-like property of opioid peptides have been made with limited success for the CNS analgesic effect due to the poor permeability across the BBB. Pharmacological approaches to enhance a peptide delivery to the CNS are to increase biochemical attributes by modifying its structure or conjugating a molecule like lipophilic enhancer, polymer, and antibody. Increasing lipophilicity has been the most useful tool for BBB penetration despite the risk of plasma protein binding [15][23][24]. DPDPE with poor BBB permeability has been modified by methylations, halogenations, Nα-acylation, and C-terminal esterification, and the resulting lipophilic analogs have produced significantly increased central analgesic effects as well as BBB permeability [23][25][26][27]. Unlike bis[Tyr-NH]-alkyl peptides, short bivalent analogs linked via an alkyl group, 2′,6′-dimethyl-L-tyrosine (Dmt)-substituted analogs produced strong analgesic effects after systemic (s.c. and oral) administrations through the MOR [28]. Among those, pyrazinone-containing analog (Figure 2) was the most potent analgesia in tail flick and hot plate tests. The molecular modeling studies suggested that the bis-Dmt analog contains the identical message and address regions and fits the MOR binding pocket, unlike large dimeric ENK or DER analogs [29]. A study showed that simple C-terminal esterification of EM can significantly increase lipophilicity, metabolic stability, and systemic antinociceptive activity after i.c.v. or oral administration, although it provided no explanation on how the change in physicochemical properties are responsible for the side effects [30].
Interestingly, [Dmt1]DALDA, a highly polar ligand with a 3+ net charge, produced 36 times more potent antinociceptive effects than morphine in mice after subcutaneous (s.c.) administration indicating high BBB transport to the brain [31]. Many studies also demonstrated that hydrophilic peptides could penetrate the cell membrane via absorptive-mediated endocytosis. The common feature was that those peptides contained multiple Arg and/or Lys. E-2078 is also a polycationic DYN A-(1-8) analog internalizing to brain capillaries by the endocytosis [32]. MOR selective DER analogs, H-Tyr-DArg-Phe-Sar/β-Ala-OH, showed potent analgesic effect with low physical and psychological dependence. Their modifications with Nα-amidinoTyr1 resulted in the slower onset of the analgesic effect after systemic administration because of the loss of a positive charge and the resulting slow BBB transport [33][34]. Based on the observation, modifying the positive charges might be a useful tool to control the BBB transport and the pharmacological effects in the CNS.
The addition of carbohydrates to a peptide, glycosylation, has also been used to improve BBB penetration and enzymatic resistance while maintaining the biological activity of a parent peptide. The applications for ENKs, DER, DLT, and EM have been successfully performed to enhance central analgesic effects after systemic administration and oral bioavailability [35][36][37][38][39][40][41]. A glycosylated hexapeptide, H-Tyr-DThr-Gly-Phe-Ile-Ser(β-DGlc)-NH2 (Figure 2), increased metabolic stability and BBB penetration as well as systemic (i.v.) antinociceptive activity, and its further modification with β-lactose and β-melibioside produced highly potent antinociception [37]. The same efforts, glucosylation or galactosylation at the C-terminal region, were made for DER and DLT and resulted in a remarkable increase of antinociceptive activity following systemic administration while retaining high in vitro activity as well [42][43]. Thr4 glycosylated analog, however, reduced activity dramatically, indicating the important role of the position for opioid activity. Orally active EM-1 analog in which a lactose moiety was linked to the N-terminus through a succinamic acid spacer showed a strong antineuropathic effect without producing constipation [41]. A cyclic glycosylated pentapeptide H-Dmt-c2,4(-SCH2CH2S-)[DCys-Aic-DPen]Ser(Glc)-NH2 possessing potent mixed MOR agonism/DOR antagonism and poor bioavailability was also successfully modified to afford high antinociceptive efficacy after intraperitoneal (i.p.) administration without acute tolerance [44]. Modeling studies and NMR analysis of glycosylated ENK analogs indicated the modification did not disturb the peptide backbone [45].
Conjugation of polyethylene glycol (PEG) has been used to enhance the therapeutic potential of opioid peptides by reducing enzymatic degradation while maintaining the biological activity of a parent peptide [15]. I.v administration of pegylated DPDPE, PEG-CH2-CH2-CO-DPDPE (Figure 2), showed an increased analgesic effect despite 176-fold lower binding affinity than DPDPE, which indicated better ability to cross the BBB and undergo hydrolysis in the brain [46]. Nonetheless, there are potential risks of losing activity and brain uptake due to increased molecular size and hydrophilicity or improper applications. To improve the brain uptake, pegylated liposomes as carriers have also been used as a carrier, and glutathione pegylated liposomal formulation of DAMGO was shown to increase and prolong the brain uptake significantly [47].
Prodrugs have been designed to improve the exposure of opioid peptides at target sites, mostly the brain, after systemic administration. Esterification of amine, hydroxyl, or carboxylic acid improves brain uptake by increasing lipophilicity. Likewise, the addition of a Phe residue to DPDPE was shown to increase the BBB permeability as a prodrug [48]. Cyclic prodrugs have also been built using linkers susceptible to esterase hydrolysis for improved delivery characteristics [49][50][51]. For a prodrug, its bioconversion to an active parent peptide within the target sites is the most critical as well as stability in the blood. A study on cyclic prodrugs of DADLE was not successful due to the formation of stable intermediate and the substrate activity for efflux transporters on the BBB nonetheless improved metabolic stability (Figure 2). The enzymatic bioconversion rates were dependent on the chirality of specific amino acids, and therefore alterations in their chirality might be critical for the success of prodrug [52].
Although oral delivery is the most preferred mode of drug administration, the bioavailability of peptides remains low due to degradation and limited absorption in the gastrointestinal tract. The development of oral delivery for opioid peptides requires the same strategies as those applied to optimize the BBB permeability, such as increasing lipophilicity and structural constraint. An amine-modified prodrug of ENK by a reversible aqueous lipidization (REAL) (Figure 2) approach produced prolonged strong oral antinociceptive effects in an inflammatory pain model along with increased metabolic stability, gastrointestinal absorption, and limited CNS-penetration [53]. Thiazole-containing cyclic DAMGO, N-terminal amidated-DERs, and phenolic group acylated-DERs are successful examples showing good oral analgesic effect [54][55]. Likewise, Nα-glycosylated EM showed a strong antinociceptive effect after oral administration in the chronic constriction injury (CCI) model but no significant constipation in contrast to morphine [41].
2.1.4. Reducing Opioid Side Effects
There appears to be no substitute for opioids in achieving adequate pain relief in some cases. MOR is primarily responsible for the antinociception but also causes the most undesirable adverse effects, limiting its clinical use. Acute administration causes respiratory depression, constipation, sedation, dizziness, and nausea, and chronic use causes tolerance, dependence, and abuse liability. Despite extensive research in the field, reducing the serious adverse effects of the MOR agonists remains unsolved. The DOR and KOR are also involved in the antinociceptive effect and related side effects, such as convulsion and dysphoria, respectively, to a lesser extent, in vivo. There have been several strategies to design opioid peptidomimetics with reduced adverse effect occurrence [56]. Those are to develop novel ligands that target multiple subtype opioid receptors, interact with receptors peripherally, produce a biased signaling, etc.
Multifunctional Ligands
Of those, multifunctional opioids that can act on more than one subtype receptor as a single molecule emerged as a promising approach to lessen the opioid-related adverse effects and provide a safer alternative to traditional opioid analgesics [57][58][59]. Mixed agonist activity at the MOR and DOR may bring synergistic antinociception that can reduce adverse effect occurrence by reducing the amount given for the same effect. It was shown that the occupation of DORs by an agonist or antagonist prevented tolerance and physical dependence on morphine [60]. Based on the involvement of DOR in MOR activity proven in numerous studies, such bifunctional ligands with a MOR agonist/DOR antagonist property as AAH8, UFP-505, KSK-103, DIPP-NH2[Ψ], [Dmt1, 2′,4′,6′-trimethyl phenylalanine (Tmp)3]-EM-2, or with a MOR/DOR agonist property as biphalin, [Dmt1, 2′-ethyl-6′-methylphenylalanine (Emp)3]-EM-2, and LYS739 seemed to be a good approach [44][57][61][62][63] (Figure 3). Biphalin is a homo-bivalent ligand consisting of two tetrapeptides and showing MOR/DOR agonist activities [64]. It produces a substantial antinociceptive effect but causes fewer side effects, suggesting synergistic effects obtained from the activation of both receptors [64][65].
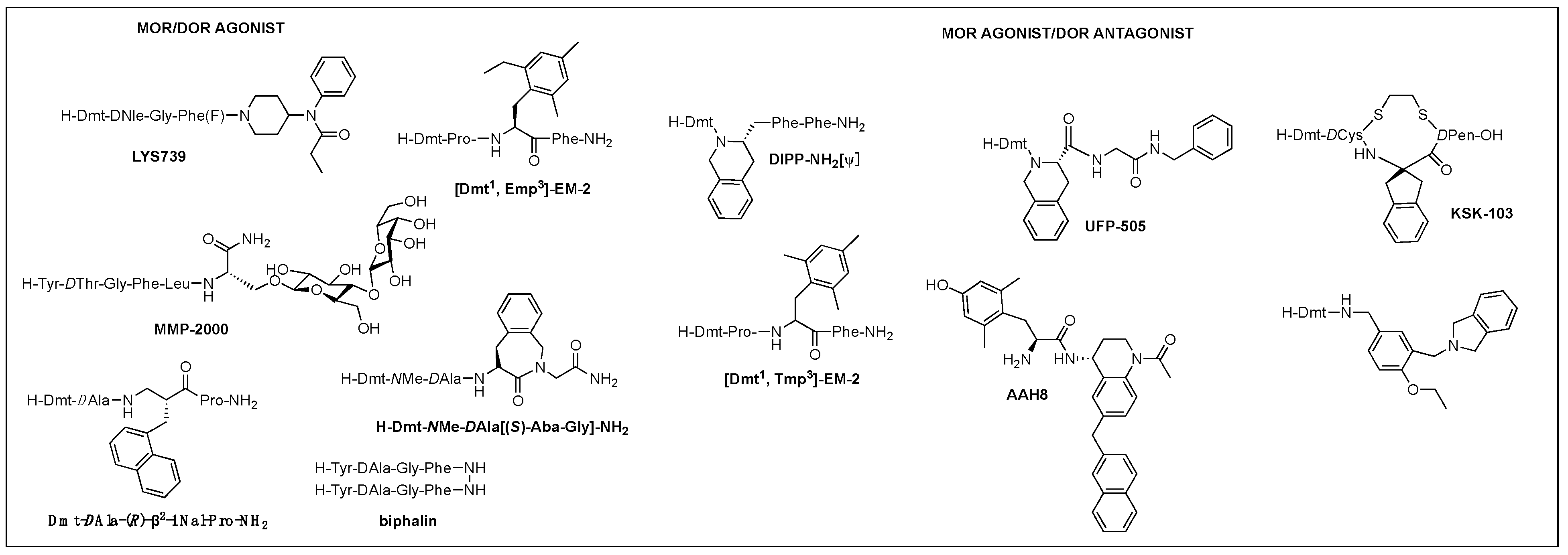
Figure 3. Multifunctional opioid peptidomimetics: MOR/DOR agonist, MOR agonist/DOR antagonist.
A glycosylated DTLES analog, NMP-2200 (YrGFLS(O-βD-lactose)-NH2) is a centrally active MOR/DOR agonist demonstrated to reduce addiction liability associated with the MOR agonist analgesics through the simultaneous activation of the MOR and DOR [38][66]. Alteration of EM-2 with a Dmt1 and an alkylated Phe3 (Dmp, Tmp, Emp) led to the bifunctional activities depending on the substitution of Phe3: di- and tri-methyl phenylalanine for the DOR antagonism and ethyl methyl phenylalanine for the DOR agonism and increased DOR affinity, yet retained MOR activity, leading to potent MOR/DOR agonism or MOR agonism/DOR antagonim [67]. Metkephamid (H-Tyr-DAla-Gly-NMeMet-NH2) is a balanced MOR/DOR agonist producing central analgesic effects after systemic administration in animal models [68][69]. It showed a lesser degree of side effects in extensive clinical tests, indicating DOR activation effect on the CNS. Clinical development, however, was stopped after phase 1 due to unusual side effects, which might be caused by the DOR activation in the CNS. For this reason, MOR agonist/DOR antagonist has been pursued more vigorously than MOR/DOR agonist.
TIPP-NH2 was the first to show a mixed MOR agonist/DOR antagonist property, and its further modifications resulted in the discovery of H-Dmt-Tic-[CH2NH]Phe-Phe-NH2 (DIPP-NH2[Ψ]) possessing a balanced MOR agonist/DOR antagonist property and exhibiting a reduced tolerance and dependence upon chronic administration [70]. Several other mixed MOR agonist/DOR antagonists containing a Dmt-Tic pharmacophore were also developed. Intrathecal (i.th.) injection of UFP-505 (H-Dmt-Tic-Gly-NH-Bzl) showed less tolerance in rats than morphine [71]. A 2-aminoindane (Aic)-substituted cyclic analog (KSK-103, Dmt-c(-SCH2CH2S-)[DCys-Aic-DPen]-OH) with a poor bioavailability also developed fewer tolerance and reward symptoms through the MOR agonist/DOR antagonist activities after C-terminal glycosylation [44][72]. Recently, numerous stable structures of MOR agonist/DOR antagonist have been developed showing central analgesic effects after systemic administration, but no in vivo study on the side effects has been reported [62][73][74][75].
Other subtype combinations are MOR/KOR, DOR/KOR, and MOR/DOR/KOR proposed for reducing side effects, but little peptidomimetic structure has been identified [57][76]. Cyclic EM-2 analog, H-Dmt-c[DLys-Phe-2′-MePhe-Asp]-NH2 was an agonist for MOR, DOR, and KOR possessing strong antinociceptive effects potentially through the concomitant activation of three receptors [77]. The substitution of β-amino acids has been shown to alter a peptide’s selectivity to a mixed receptor property. Likewise, modifications of a MOR selective morphiceptin with β2- or β3-amino acid residues resulted in various multifunctional opioid profiles. Dmt-DAla-(R)-β2-1-Nal-Pro-NH2 was a potent MOR/DOR/KOR agonist exhibiting a strong peripheral antinociceptive effect after i.p. and oral administration [78].
Localization of the Site: Peripherally Mediated Analgesics
Emerging evidence indicates that opioids also act in the periphery to contribute to analgesic actions, although much less is known about this, compared to the central functions [56][79]. During inflammation, peripheral opioids interact with upregulated opioid receptors in damaged tissue, and the localized interactions can induce therapeutically safe analgesic effects by avoiding undesirable centrally mediated adverse effects [56][79]. The main strategy to localize the interactions in the PNS was to increase the hydrophilicity of the opioid peptides to inhibit the brain uptake. It afforded numerous peripherally acting opioid peptidomimetics with a variety of biological profiles [80].
[β-Pro2]-EM-1 is a potent MOR agonist showing peripheral antinociceptive activity after systemic administration along with increased metabolic stability [81]. DAMGO, CR845 (Difelikefalin, H-DPhe-DPhe-DLeu-DLys-[γ-(4-N-piperidinyl)amino carboxylic acid]), DALDA, PL017 ([NMePhe3, DPro4]morphiceptin), CJC-1008, and BW443C (H-Tyr-DArg-Gly-Phe(p-NO2)-Pro-NH2 were shown to produce peripherally localized analgesic effects [82][83][84]. Preliminary clinical studies of BW443C demonstrated peripheral opioid analgesic effects avoiding relevant central effects [85]. CR845, the first approved opioid peptide drug, is in medical use of moderate to severe itching and is under development for the treatment of postoperative and osteoarthritis pain [86]. CJC-1008, a maleimido propionyl group-attached DYN A-(1-13) analog, was designed to promote covalent bond formation with serum albumin and showed a greater peripheral analgesic effect compared to placebo with prolonged activity in Phase II clinical study [83]. Likewise, the conjugation of a biocompatible nano-carrier with a big molecular size also localizes the opioid peptides by blocking the BBB transport [20][56][79].
Biased Ligands, etc.
In β-arrestin-2 knockout mice, morphine enhanced analgesia with reduced constipation and respiratory depression [87]. Based on the result, biased ligands that preferentially activate one downstream pathway, G-protein pathway over β-arrestin, have been suggested as a safer, better tolerated, and more efficacious opioid analgesic despite a recent study to the contrary [88][89][90][91]. A study discovered a cyclic peptide, H-Dmt-c[DLys-Phe(p-CF3)-Phe-Asp]-NH2, with high MOR affinity and in vitro functional activity, which turned out to be a G-protein biased MOR agonist [92]. Studies suggested positions 4 and 5 of ENK might be responsible for the biased signaling because the substitutions at the positions resulted in distinctly biased signaling [93][94][95]. Furthermore, positive allosteric modulators were shown to enhance the activities of endogenous opioid peptides, maintain their temporal and spatial action, and potentially limit the adverse effects [96]. Although a few allosteric modulators were identified for opioid receptors and characterized in vitro, their utility in vivo is yet to be determined [97].
2.2. Structural Aspect
2.2.1. Conformational Studies and Design from Pharmacophore
The hierarchical approach to peptidomimetics is to (i) identify the conformational structure along with the side chain functional requirements for a target receptor, (ii) reflect the outcomes in building a constrained structure, and (iii) determine the three-dimensional arrangement of the critical side chain and backbone functionalities. Based on this, constrained peptidomimetics in which the peptide scaffold is replaced globally or locally (at a particular amino acid residue or peptide bond) by other organic moieties are designed and synthesized [19][98]. The use of constrained peptidomimetics, new insight into their interactions with opioid receptors, and additional fine-tuning of the constrained structure are critical in developing a novel therapeutic analgesic agent. Recent advances in structural biology using crystallography, molecular docking, molecular dynamics simulations, and NMR spectroscopy provided key insights into the binding modes of opioid peptidomimetics to the receptors. Interestingly, a study using cryo-electron microscopy indicated that the N-terminus of DAMGO interact with conserved receptor residue of the morphinan ligand pocket while the C-terminus occupies the regions for MOR selectivity [4].
Regardless of the sequences, natural opioid peptides contain multiple aromatic amino acids in common as key pharmacophoric residues, and their SARs have long been analyzed based on the message–address concept (Figure 4). The message region of opioid peptides is the N-terminal three or tetrapeptide responsible for biological activity, and the address region is the variable structure following the message region responsible for receptor selectivity [99][100][101][102]. In the message region, Tyr and Phe are key pharmacophoric residues, and their Nα-amino, phenolic, and aromatic groups are known to be critical for receptor recognition. Relative orientation and length of the two aromatic rings are also critical for the subtype selectivity. Overall longer backbones are preferred for MOR and shorter ones for DOR. DOR or KOR selectivity over the other subtypes is attributed to the C-terminal region, as shown in DLT (the hydrophobic Val5-Val6 residues) and DYN A (the basic Arg6-Lys11 residues), respectively. The message–address concept has contributed to the design of highly potent and selective opioid peptides and their modifications for the development of novel opioid peptidomimetics [102][103][104][105].
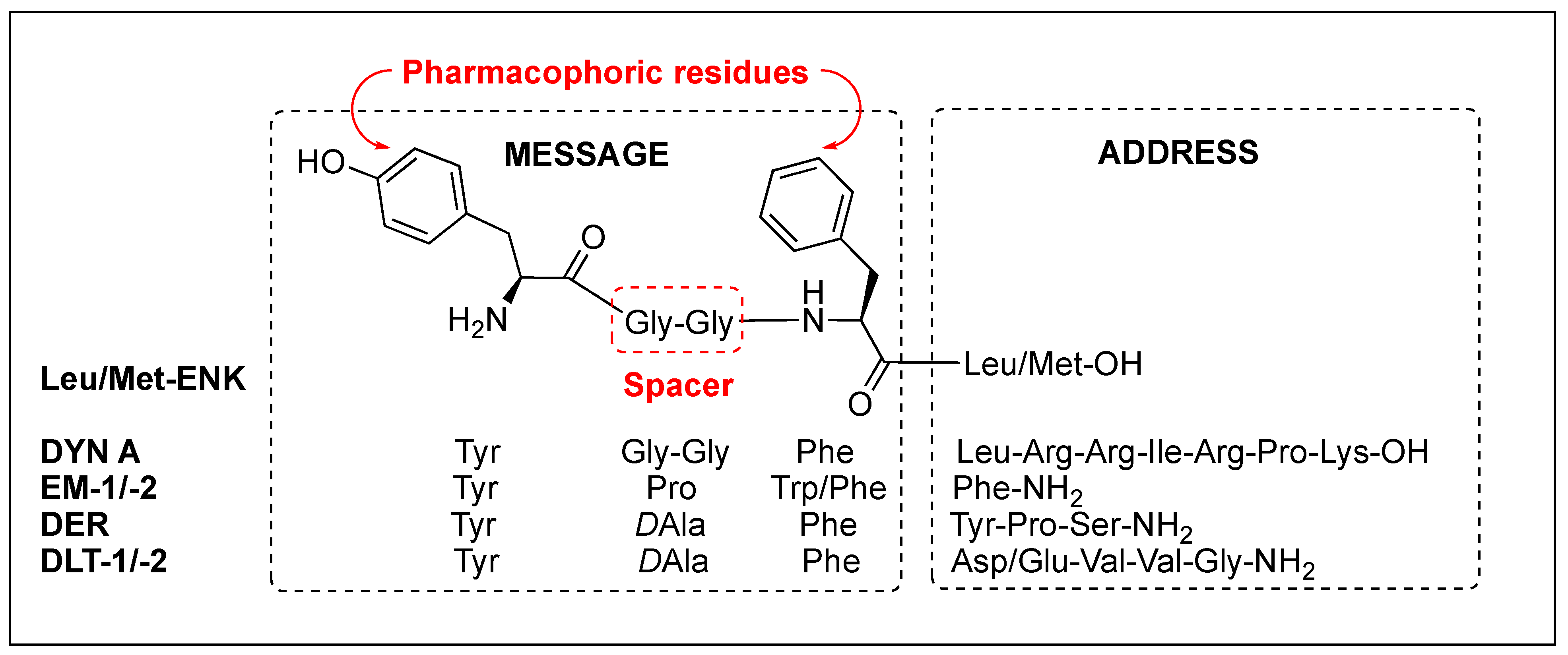
Figure 4. Message and address regions of natural opioid peptides.
This entry is adapted from the peer-reviewed paper 10.3390/biom12091241
References
- Eguchi, M. Recent advances in selective opioid receptor agonists and antagonists. Med. Res. Rev. 2004, 24, 182–212.
- Onogi, T.; Minami, M.; Katao, Y.; Nakagawa, T.; Aoki, Y.; Toya, T.; Katsumata, S.; Satoh, M. DAMGO, a μ-opioid receptor selective agonist, distinguishes between μ-and δ-opioid receptors around their first extracellular loops. FEBS Lett. 1995, 357, 93–97.
- Handa, B.K.; Land, A.C.; Lord, J.A.; Morgan, B.A.; Rance, M.J.; Smith, C.F. Analogues of beta-LPH61-64 possessing selective agonist activity at mu-opiate receptors. Eur. J. Pharmacol. 1981, 70, 531–540.
- Koehl, A.; Hu, H.; Maeda, S.; Zhang, Y.; Qu, Q.; Paggi, J.M.; Latorraca, N.R.; Hilger, D.; Dawson, R.; Matile, H.; et al. Structure of the µ-opioid receptor–Gi protein complex. Nature 2018, 558, 547–552.
- Mosberg, H.I.; Hurst, R.; Hruby, V.J.; Gee, K.; Yamamura, H.I.; Galligan, J.J.; Burks, T.F. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc. Natl. Acad. Sci. USA 1983, 80, 5871–5874.
- Wang, Y.; Kuczera, K. Molecular dynamics simulations of cyclic and linear DPDPE: Influence of the disulfide bond on peptide flexibility. J. Phys. Chem. 1996, 100, 2555–2563.
- Lung, F.D.; Meyer, J.P.; Lou, B.S.; Xiang, L.; Li, G.; Davis, P.; DeLeon, I.A.; Yamamura, H.I.; Porreca, F.; Hruby, V.J. Effects of modifications of residues in position 3 of dynorphin A(1-11)-NH2 on kappa receptor selectivity and potency. J. Med. Chem. 1996, 39, 2456–2460.
- Li, T.; Jinsmaa, Y.; Nedachi, M.; Miyazaki, A.; Tsuda, Y.; Ambo, A.; Sasaki, Y.; Bryant, S.D.; Marczak, E.; Li, Q.; et al. Transformation of mu-opioid receptor agonists into biologically potent mu-opioid receptor antagonists. Bioorg. Med. Chem. 2007, 15, 1237–1251.
- Fichna, J.; do-Rego, J.-C.; Kosson, P.; Costentin, J.; Janecka, A. Characterization of antinociceptive activity of novel endomorphin-2 and morphiceptin analogs modified in the third position. Biochem. Pharmacol. 2005, 69, 179–185.
- Schiller, P.W.; Berezowska, I.; Nguyen, T.M.-D.; Schmidt, R.; Lemieux, C.; Chung, N.N.; Falcone-Hindley, M.L.; Yao, W.; Liu, J.; Iwama, S. Novel ligands lacking a positive charge for the δ-and μ-opioid receptors. J. Med. Chem. 2000, 43, 551–559.
- Gairin, J.E.; Mazarguil, H.; Alvinerie, P.; Botanch, C.; Cros, J.; Meunier, J.C. N,N-diallyl-tyrosyl substitution confers antagonist properties on the kappa-selective opioid peptide dynorphin A(1-11). Br. J. Pharmacol. 1988, 95, 1023–1030.
- Schiller, P.W.; Weltrowska, G.; Nguyen, T.M.-D.; Lemieux, C.; Chung, N.N.; Lu, Y. Conversion of δ-, κ-and μ-Receptor Selective Opioid Peptide Agonists into δ-, κ-and μ-Selective Antagonists. Life Sci. 2003, 73, 691–698.
- Tancredi, T.; Salvadori, S.; Amodeo, P.; Picone, D.; Lazarus, L.H.; Bryant, S.D.; Guerrini, R.; Marzola, G.; Temussi, P.A. Conversion of enkephalin and dermorphin into delta-selective opioid antagonists by single-residue substitution. Eur. J. Biochem. 1994, 224, 241–247.
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Sign. Trans. Targeted Therap. 2022, 7, 1–27.
- Witt, K.A.; Davis, T.P. CNS drug delivery: Opioid peptides and the blood-brain barrier. AAPS J. 2006, 8, E76–E88.
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707.
- Gentilucci, L.; De Marco, R.; Cerisoli, L. Chemical modifications designed to improve peptide stability: Incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203.
- Pert, C.B.; Pert, A.; Chang, J.K.; Fong, B.T. (D-Ala2)-Met-enkephalinamide: A potent, long-lasting synthetic pentapeptide analgesic. Science 1976, 194, 330–332.
- Avan, I.; Hall, C.D.; Katritzky, A.R. Peptidomimetics via modifications of amino acids and peptide bonds. Chem. Soc. Rev. 2014, 43, 3575–3594.
- Roberts, L.R.; Brady, K.; Brown, A.; Davey, D.; Feng, L.; Huang, H.; Liu, D.; Malet, L.; McMurray, G.; Phelan, A.; et al. Kappa agonist CovX-Bodies. Bioorg. Med. Chem. Lett. 2012, 22, 4173–4178.
- Kropotova, E.S.; Ivleva, I.S.; Karpenko, M.N.; Mosevitsky, M.I. Design of enkephalin modifications protected from brain extracellular peptidases providing long-term analgesia. Bioorg. Med. Chem. 2020, 28, 115184.
- Beaudeau, J.L.; Blais, V.; Holleran, B.J.; Bergeron, A.; Pineyro, G.; Guerin, B.; Gendron, L.; Dory, Y.L. N-Guanidyl and C-Tetrazole Leu-Enkephalin Derivatives: Efficient Mu and Delta Opioid Receptor Agonists with Improved Pharmacological Properties. ACS Chem. Neurosci. 2019, 10, 1615–1626.
- Weber, S.J.; Abbruscato, T.J.; Brownson, E.A.; Lipkowski, A.W.; Polt, R.; Misicka, A.; Haaseth, R.C.; Bartosz, H.; Hruby, V.J.; Davis, T.P. Assessment of an in vitro blood-brain barrier model using several enkephalin opioid analogs. J. Pharmacol. Exp. Ther. 1993, 266, 1649–1655.
- Banks, W.A.; Kastin, A.J. Peptides and the blood-brain barrier: Lipophilicity as a predictor of permeability. Brain Res. Bullet. 1985, 15, 287–292.
- Hansen, D.W., Jr.; Stapelfeld, A.; Savage, M.A.; Reichman, M.; Hammond, D.L.; Haaseth, R.C.; Mosberg, H.I. Systemic analgesic activity and delta-opioid selectivity in enkephalin. J. Med. Chem. 1992, 35, 684–687.
- Weber, S.J.; Greene, D.L.; Sharma, S.D.; Yamamura, H.I.; Kramer, T.H.; Burks, T.F.; Hruby, V.J.; Hersh, L.B.; Davis, T.P. Distribution and analgesia of enkephalin and two halogenated analogs after intravenous administration. J. Pharmacol. Exp. Ther. 1991, 259, 1109–1117.
- Chandrakumar, N.S.; Stapelfeld, A.; Beardsley, P.M.; Lopez, O.T.; Drury, B.; Anthony, E.; Savage, M.A.; Williamson, L.N.; Reichman, M. Analogs of the. delta. opioid receptor selective cyclic peptide -enkephalin: 2’, 6’-dimethyltyrosine and Gly3-Phe4 amide bond isostere substitutions. J. Med. Chem. 1992, 35, 2928–2938.
- Jinsmaa, Y.; Miyazaki, A.; Fujita, Y.; Li, T.; Fujisawa, Y.; Shiotani, K.; Tsuda, Y.; Yokoi, T.; Ambo, A.; Sasaki, Y.; et al. Oral bioavailability of a new class of micro-opioid receptor agonists containing 3,6-bis-2(1H)-pyrazinone with central-mediated analgesia. J. Med. Chem. 2004, 47, 2599–2610.
- Okada, Y.; Tsuda, Y.; Fujita, Y.; Yokoi, T.; Sasaki, Y.; Ambo, A.; Konishi, R.; Nagata, M.; Salvadori, S.; Jinsmaa, Y. Unique high-affinity synthetic μ-opioid receptor agonists with central-and systemic-mediated analgesia. J. Med. Chem. 2003, 46, 3201–3209.
- Wang, C.L.; Qiu, T.T.; Yang, D.J.; Yuan, B.Y.; Han, F.T.; Li, L.; Gu, N. Endomorphin-2 analogs with C-terminal esterification produce potent systemic antinociception with reduced tolerance and gastrointestinal side effects. Neuropharmacology 2017, 116, 98–109.
- Zhao, K.; Luo, G.; Zhao, G.M.; Schiller, P.W.; Szeto, H.H. Transcellular transport of a highly polar 3+ net charge opioid tetrapeptide. J. Pharmacol. Exp. Ther. 2003, 304, 425–432.
- Terasaki, T.; Hirai, K.; Sato, H.; Kang, Y.; Tsuji, A. Absorptive-mediated endocytosis of a dynorphin-like analgesic peptide, E-2078 into the blood-brain barrier. J. Pharmacol. Exp. Therap. 1989, 251, 351–357.
- Deguchi, Y.; Miyakawa, Y.; Sakurada, S.; Naito, Y.; Morimoto, K.; Ohtsuki, S.; Hosoya, K.I.; Terasaki, T. Blood–brain barrier transport of a novel µ1-specific opioid peptide, H-Tyr-d-Arg-Phe-β-Ala-OH (TAPA). J. Neurochem. 2003, 84, 1154–1161.
- Deguchi, Y.; Naito, Y.; Ohtsuki, S.; Miyakawa, Y.; Morimoto, K.; Hosoya, K.-I.; Sakurada, S.; Terasaki, T. Blood-brain barrier permeability of novel dermorphin (1–4) analogs: Transport property is related to the slow onset of antinociceptive activity in the central nervous system. J. Pharmacol. Exp. Therap. 2004, 310, 177–184.
- Polt, R.; Dhanasekaran, M.; Keyari, C.M. Glycosylated neuropeptides: A new vista for neuropsychopharmacology? Med. Res. Rev. 2005, 25, 557–585.
- Egleton, R.D.; Mitchell, S.A.; Huber, J.D.; Janders, J.; Stropova, D.; Polt, R.; Yamamura, H.I.; Hruby, V.J.; Davis, T.P. Improved bioavailability to the brain of glycosylated Met-enkephalin analogs. Brain Res. 2000, 881, 37–46.
- Egleton, R.D.; Mitchell, S.A.; Huber, J.D.; Palian, M.M.; Polt, R.; Davis, T.P. Improved blood-brain barrier penetration and enhanced analgesia of an opioid peptide by glycosylation. J. Pharmacol. Exp. Ther. 2001, 299, 967–972.
- Li, Y.; Lefever, M.R.; Muthu, D.; Bidlack, J.M.; Bilsky, E.J.; Polt, R. Opioid glycopeptide analgesics derived from endogenous enkephalins and endorphins. Fut. Med. Chem. 2012, 4, 205–226.
- Fichna, J.; Mazur, M.; Grzywacz, D.; Kamysz, W.; Perlikowska, R.; Piekielna, J.; Sobczak, M.; Salaga, M.; Toth, G.; Janecka, A.; et al. Novel glycosylated endomorphin-2 analog produces potent centrally-mediated antinociception in mice after peripheral administration. Bioorg. Med. Chem. Lett. 2013, 23, 6673–6676.
- Ballet, S.; Betti, C.; Novoa, A.; Tomboly, C.; Nielsen, C.U.; Helms, H.C.; Lesniak, A.; Kleczkowska, P.; Chung, N.N.; Lipkowski, A.W.; et al. In Vitro Membrane Permeation Studies and in Vivo Antinociception of Glycosylated Dmt(1)-DALDA Analogues. ACS Med. Chem. Lett. 2014, 5, 352–357.
- Varamini, P.; Mansfeld, F.M.; Blanchfield, J.T.; Wyse, B.D.; Smith, M.T.; Toth, I. Synthesis and biological evaluation of an orally active glycosylated endomorphin-1. J. Med. Chem. 2012, 55, 5859–5867.
- Tomatis, R.; Marastoni, M.; Balboni, G.; Guerrini, R.; Capasso, A.; Sorrentino, L.; Santagada, V.; Caliendo, G.; Lazarus, L.H.; Salvadori, S. Synthesis and pharmacological activity of deltorphin and dermorphin-related glycopeptides. J. Med. Chem. 1997, 40, 2948–2952.
- Negri, L.; Lattanzi, R.; Tabacco, F.; Orru, L.; Severini, C.; Scolaro, B.; Rocchi, R. Dermorphin and deltorphin glycosylated analogues: Synthesis and antinociceptive activity after systemic administration. J. Med. Chem. 1999, 42, 400–404.
- Mosberg, H.I.; Yeomans, L.; Anand, J.P.; Porter, V.; Sobczyk-Kojiro, K.; Traynor, J.R.; Jutkiewicz, E.M. Development of a bioavailable mu opioid receptor (MOPr) agonist, delta opioid receptor (DOPr) antagonist peptide that evokes antinociception without development of acute tolerance. J. Med. Chem. 2014, 57, 3148–3153.
- Palian, M.M.; Boguslavsky, V.I.; O’Brien, D.F.; Polt, R. Glycopeptide-membrane interactions: Glycosyl enkephalin analogues adopt turn conformations by NMR and CD in amphipathic media. J. Am. Chem. Soc. 2003, 125, 5823–5831.
- Witt, K.A.; Huber, J.D.; Egleton, R.D.; Roberts, M.J.; Bentley, M.D.; Guo, L.; Wei, H.; Yamamura, H.I.; Davis, T.P. Pharmacodynamic and pharmacokinetic characterization of poly(ethylene glycol) conjugation to met-enkephalin analog -enkephalin (DPDPE). J. Pharmacol. Exp. Ther. 2001, 298, 848–856.
- Lindqvist, A.; Rip, J.; Gaillard, P.J.; Bjorkman, S.; Hammarlund-Udenaes, M. Enhanced brain delivery of the opioid peptide DAMGO in glutathione pegylated liposomes: A microdialysis study. Mol. Pharm. 2013, 10, 1533–1541.
- Greene, D.L.; Hau, V.S.; Abbruscato, T.J.; Bartosz, H.; Misicka, A.; Lipkowski, A.W.; Hom, S.; Gillespie, T.J.; Hruby, V.J.; Davis, T.P. Enkephalin analog prodrugs: Assessment of in vitro conversion, enzyme cleavage characterization and blood-brain barrier permeability. J. Pharmacol. Exp. Ther. 1996, 277, 1366–1375.
- Ouyang, H.; Tang, F.; Siahaan, T.J.; Borchardt, R.T. A modified coumarinic acid-based cyclic prodrug of an opioid peptide: Its enzymatic and chemical stability and cell permeation characteristics. Pharm. Res. 2002, 19, 794–801.
- Yang, J.Z.; Chen, W.; Borchardt, R.T. In vitro stability and in vivo pharmacokinetic studies of a model opioid peptide, H-Tyr-D-Ala-Gly-Phe-D-Leu-OH (DADLE), and its cyclic prodrugs. J. Pharmacol. Exp. Ther. 2002, 303, 840–848.
- Prokai-Tatrai, K.; Kim, H.-S.; Prokai, L. The utility of oligopeptidase in brain-targeting delivery of an enkephalin analogue by prodrug design. Open Med. Chem. J. 2008, 2, 97.
- Liederer, B.M.; Borchardt, R.T. Stability of oxymethyl-modified coumarinic acid cyclic prodrugs of diastereomeric opioid peptides in biological media from various animal species including human. J. Pharm. Sci. 2005, 94, 2198–2206.
- Wang, J.; Hogenkamp, D.J.; Tran, M.; Li, W.-Y.; Yoshimura, R.F.; Johnstone, T.B.; Shen, W.-C.; Gee, K.W. Reversible lipidization for the oral delivery of leu-enkephalin. J. Drug Target. 2006, 14, 127–136.
- Ogawa, T.; Araki, M.; Miyamae, T.; Okayama, T.; Hagiwara, M.; Sakurada, S.; Morikawa, T. Synthesis and antinociceptive activity of orally active opioid peptides: Improvement of oral bioavailability by esterification. Chem. Pharm. Bull. 2003, 51, 759–771.
- Ogawa, T.; Miyamae, T.; Murayama, K.; Okuyama, K.; Okayama, T.; Hagiwara, M.; Sakurada, S.; Morikawa, T. Synthesis and structure-activity relationships of an orally available and long-acting analgesic peptide, N(alpha)-amidino-Tyr-D-Arg-Phe-MebetaAla-OH (ADAMB). J. Med. Chem. 2002, 45, 5081–5089.
- Machelska, H.; Celik, M.O. Advances in Achieving Opioid Analgesia Without Side Effects. Front. Pharmacol. 2018, 9, 1388.
- Anand, J.P.; Montgomery, D. Multifunctional Opioid Ligands. Handb. Exp. Pharmacol. 2018, 247, 21–51.
- Hruby, V.J. Multivalent peptide and peptidomimetic ligands for the treatment of pain without toxicities and addiction. Peptides 2019, 116, 63–67.
- Dietis, N.; Guerrini, R.; Calo, G.; Salvadori, S.; Rowbotham, D.J.; Lambert, D.G. Simultaneous targeting of multiple opioid receptors: A strategy to improve side-effect profile. Br. J. Anaesth 2009, 103, 38–49.
- Zhu, Y.; King, M.A.; Schuller, A.G.; Nitsche, J.F.; Reidl, M.; Elde, R.P.; Unterwald, E.; Pasternak, G.W.; Pintar, J.E. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron 1999, 24, 243–252.
- Mosberg, H.I.; Yeomans, L.; Harland, A.A.; Bender, A.M.; Sobczyk-Kojiro, K.; Anand, J.P.; Clark, M.J.; Jutkiewicz, E.M.; Traynor, J.R. Opioid peptidomimetics: Leads for the design of bioavailable mixed efficacy mu opioid receptor (MOR) agonist/delta opioid receptor (DOR) antagonist ligands. J. Med. Chem. 2013, 56, 2139–2149.
- Henry, S.; Anand, J.P.; Twarozynski, J.J.; Brinkel, A.C.; Pogozheva, I.D.; Sears, B.F.; Jutkiewicz, E.M.; Traynor, J.R.; Mosberg, H.I. Aromatic–Amine Pendants Produce Highly Potent and Efficacious Mixed Efficacy μ-Opioid Receptor (MOR)/δ-Opioid Receptor (DOR) Peptidomimetics with Enhanced Metabolic Stability. J. Med. Chem. 2020, 63, 1671–1683.
- Lee, Y.S.; Kulkarani, V.; Cowell, S.M.; Ma, S.W.; Davis, P.; Hanlon, K.E.; Vanderah, T.W.; Lai, J.; Porreca, F.; Vardanyan, R.; et al. Development of potent mu and delta opioid agonists with high lipophilicity. J. Med. Chem. 2011, 54, 382–386.
- Cowell, S.M.; Lee, Y.S. Biphalin: The Foundation of Bivalent Ligands. Curr. Med. Chem. 2016, 23, 3267–3284.
- Horan, P.J.; Mattia, A.; Bilsky, E.J.; Weber, S.; Davis, T.P.; Yamamura, H.I.; Malatynska, E.; Appleyard, S.M.; Slaninova, J.; Misicka, A.; et al. Antinociceptive profile of biphalin, a dimeric enkephalin analog. J. Pharmacol. Exp. Ther. 1993, 265, 1446–1454.
- Lowery, J.J.; Raymond, T.J.; Giuvelis, D.; Bidlack, J.M.; Polt, R.; Bilsky, E.J. In vivo characterization of MMP-2200, a mixed delta/mu opioid agonist, in mice. J. Pharmacol. Exp. Ther. 2011, 336, 767–778.
- Li, T.; Shiotani, K.; Miyazaki, A.; Tsuda, Y.; Ambo, A.; Sasaki, Y.; Jinsmaa, Y.; Marczak, E.; Bryant, S.D.; Lazarus, L.H.; et al. Bifunctional endomorphin-2 analogues substituted at position 3 with alkylated phenylalanine derivatives yield potent mixed mu-agonist/delta-antagonist and dual mu-agonist/delta-agonist opioid ligands. J. Med. Chem. 2007, 50, 2753–2766.
- Frederickson, R.C.; Smithwick, E.L.; Shuman, R.; Bemis, K.G. Metkephamid, a systemically active analog of methionine enkephalin with potent opioid alpha-receptor activity. Science 1981, 211, 603–605.
- Burkhardt, C.; Frederickson, R.C.; Pasternak, G.W. Metkephamid (Tyr-D-ala-Gly-Phe-N(Me)Met-NH2), a potent opioid peptide: Receptor binding and analgesic properties. Peptides 1982, 3, 869–871.
- Schiller, P.W.; Schmidt, R.; Weltrowska, G.; Berezowska, I.; Nguyen, T.M.-D.; Dupuis, S.; Chung, N.N.; Lemieux, C.; Wilkes, B.C.; Carpenter, K.A. Conformationally constrained opioid peptide analogs with novel activity profiles. Lett. Peptide Sci. 1998, 5, 209–214.
- Dietis, N.; McDonald, J.; Molinari, S.; Calo, G.; Guerrini, R.; Rowbotham, D.J.; Lambert, D.G. Pharmacological characterization of the bifunctional opioid ligand H-Dmt-Tic-Gly-NH-Bzl (UFP-505). Br. J. Anaesth 2012, 108, 262–270.
- Purington, L.C.; Sobczyk-Kojiro, K.; Pogozheva, I.D.; Traynor, J.R.; Mosberg, H.I. Development and in vitro characterization of a novel bifunctional mu-agonist/delta-antagonist opioid tetrapeptide. ACS Chem. Biol. 2011, 6, 1375–1381.
- Harland, A.A.; Yeomans, L.; Griggs, N.W.; Anand, J.P.; Pogozheva, I.D.; Jutkiewicz, E.M.; Traynor, J.R.; Mosberg, H.I. Further optimization and evaluation of bioavailable, mixed-efficacy μ-opioid receptor (MOR) agonists/δ-opioid receptor (DOR) antagonists: Balancing MOR and DOR affinities. J. Med. Chem. 2015, 58, 8952–8969.
- Henry, S.P.; Fernandez, T.J.; Anand, J.P.; Griggs, N.W.; Traynor, J.R.; Mosberg, H.I. Structural Simplification of a Tetrahydroquinoline-Core Peptidomimetic mu-Opioid Receptor (MOR) Agonist/delta-Opioid Receptor (DOR) Antagonist Produces Improved Metabolic Stability. J. Med. Chem. 2019, 62, 4142–4157.
- Bender, A.M.; Clark, M.J.; Agius, M.P.; Traynor, J.R.; Mosberg, H.I. Synthesis and evaluation of 4-substituted piperidines and piperazines as balanced affinity mu opioid receptor (MOR) agonist/delta opioid receptor (DOR) antagonist ligands. Bioorg. Med. Chem. Lett. 2014, 24, 548–551.
- Lee, Y.S.; Remesic, M.; Ramos-Colon, C.; Wu, Z.; LaVigne, J.; Molnar, G.; Tymecka, D.; Misicka, A.; Streicher, J.M.; Hruby, V.J. Multifunctional Enkephalin Analogs with a New Biological Profile: MOR/DOR Agonism and KOR Antagonism. Biomedicines 2021, 9, 625.
- Perlikowska, R.; Malfacini, D.; Cerlesi, M.C.; Piekielna, J.; Floriot, L.; Henry, T.; Do-Rego, J.C.; Tömböly, C.; Kluczyk, A.; Janecka, A. Pharmacological characterization of endomorphin-2-based cyclic pentapeptides with methylated phenylalanine residues. Peptides 2014, 55, 145–150.
- Gach-Janczak, K.; Piekielna-Ciesielska, J.; Adamska-Bartłomiejczyk, A.; Perlikowska, R.; Kruszyński, R.; Kluczyk, A.; Krzywik, J.; Sukiennik, J.; Cerlesi, M.C.; Calo, G. Synthesis and activity of opioid peptidomimetics with β2-and β3-amino acids. Peptides 2017, 95, 116–123.
- Martinez, V.; Abalo, R. Peripherally acting opioid analgesics and peripherally-induced analgesia. Behav. Pharmacol. 2020, 31, 136–158.
- Zaitseva, N.; Galan, S.; Pavlova, L. Prospects of a search for kappa-opioid receptor agonists with analgesic activity. Pharm. Chem. J. 2018, 51, 843–851.
- Spampinato, S.; Qasem, A.R.; Calienni, M.; Murari, G.; Gentilucci, L.; Tolomelli, A.; Cardillo, G. Antinociception by a peripherally administered novel endomorphin-1 analogue containing beta-proline. Eur. J. Pharmacol. 2003, 469, 89–95.
- Hesselink, J.M.K. CR845 (Difelikefalin), A Kappa Receptors Agonist In Phase III By CARA Therapeutics: A Case Of ‘Spin’In Scientific Writing? J. Pharmacol. Clin. Res. 2017, 2, 555588.
- Wallace, M.S.; Moulin, D.; Clark, A.; Wasserman, R.; Neale, A.; Morley-Forster, P.; Castaigne, J.-P.; Teichman, S. A Phase II, multicenter, randomized, double-blind, placebo-controlled crossover study of CJC-1008—a long-acting, parenteral opioid analgesic—in the treatment of postherpetic neuralgia. J. Opioid. Manag. 2006, 2, 167–173.
- Tiwari, V.; Yang, F.; He, S.Q.; Shechter, R.; Zhang, C.; Shu, B.; Zhang, T.; Tiwari, V.; Wang, Y.; Dong, X.; et al. Activation of Peripheral mu-opioid Receptors by Dermorphin (1-4) Amide Leads to Modality-preferred Inhibition of Neuropathic Pain. Anesthesiology 2016, 124, 706–720.
- Posner, J.; Moody, S.; Peck, A.; Rutter, D.; Telekes, A. Analgesic, central, cardiovascular and endocrine effects of the enkephalin analogue Tyr-D. Arg-Gly-Phe (4NO 2)-Pro-NH 2 (443C81) in healthy volunteers. Eur. J. Clin. Pharmacol. 1990, 38, 213–218.
- Deeks, E.D. Difelikefalin: First Approval. Drugs 2021, 81, 1937–1944.
- DeWire, S.M.; Yamashita, D.S.; Rominger, D.H.; Liu, G.; Cowan, C.L.; Graczyk, T.M.; Chen, X.T.; Pitis, P.M.; Gotchev, D.; Yuan, C.; et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J. Pharmacol. Exp. Ther. 2013, 344, 708–717.
- Piekielna-Ciesielska, J.; Wtorek, K.; Janecka, A. Biased Agonism as an Emerging Strategy in the Search for Better Opioid Analgesics. Curr. Med. Chem. 2020, 27, 1562–1575.
- He, L.; Gooding, S.W.; Lewis, E.; Felth, L.C.; Gaur, A.; Whistler, J.L. Pharmacological and genetic manipulations at the µ-opioid receptor reveal arrestin-3 engagement limits analgesic tolerance and does not exacerbate respiratory depression in mice. Neuropsychopharmacology 2021, 46, 2241–2249.
- Faouzi, A.; Varga, B.R.; Majumdar, S. Biased opioid ligands. Molecules 2020, 25, 4257.
- Madariaga-Mazón, A.; Marmolejo-Valencia, A.F.; Li, Y.; Toll, L.; Houghten, R.A.; Martinez-Mayorga, K. Mu-Opioid receptor biased ligands: A safer and painless discovery of analgesics? Drug Discov. Today 2017, 22, 1719–1729.
- Piekielna-Ciesielska, J.; Ferrari, F.; Calo, G.; Janecka, A. Cyclopeptide Dmt-NH2, a novel G protein-biased agonist of the mu opioid receptor. Peptides 2018, 101, 227–233.
- Bella Ndong, D.; Blais, V.; Holleran, B.J.; Proteau-Gagné, A.; Cantin-Savoie, I.; Robert, W.; Nadon, J.F.; Beauchemin, S.; Leduc, R.; Piñeyro, G. Exploration of the fifth position of leu-enkephalin and its role in binding and activating delta (DOP) and mu (MOP) opioid receptors. Peptide Sci. 2019, 111, e24070.
- Sharma, K.K.; Cassell, R.J.; Su, H.; Blaine, A.T. Modulating β Arrestin-2 Recruitment at the δ- and µ-Opioid Receptors; Cambridge Open Engage: Cambridge, UK, 2020.
- Cassell, R.J.; Sharma, K.K.; Su, H.; Cummins, B.R.; Cui, H.; Mores, K.L.; Blaine, A.T.; Altman, R.A.; van Rijn, R.M. The Meta-Position of Phe4 in Leu-enkephalin Regulates Potency, Selectivity, Functional Activity, and Signaling Bias at the Delta and Mu Opioid Receptors. Molecules 2019, 24, 4542.
- Kandasamy, R.; Hillhouse, T.M.; Livingston, K.E.; Kochan, K.E.; Meurice, C.; Eans, S.O.; Li, M.-H.; White, A.D.; Roques, B.P.; McLaughlin, J.P. Positive allosteric modulation of the mu-opioid receptor produces analgesia with reduced side effects. Proc. Natl. Acad. Sci. USA 2021, 118, e2000017118.
- Remesic, M.; Hruby, V.J.; Porreca, F.; Lee, Y.S. Recent Advances in the Realm of Allosteric Modulators for Opioid Receptors for Future Therapeutics. ACS Chem. Neurosci. 2017, 8, 1147–1158.
- Cowell, S.M.; Lee, Y.S.; Cain, J.P.; Hruby, V.J. Exploring Ramachandran and chi space: Conformationally constrained amino acids and peptides in the design of bioactive polypeptide ligands. Curr. Med. Chem. 2004, 11, 2785–2798.
- Yamazaki, T.; Ro, S.; Goodman, M.; Chung, N.N.; Schiller, P.W. A topochemical approach to explain morphiceptin bioactivity. J. Med. Chem. 1993, 36, 708–719.
- Granier, S.; Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Weis, W.I.; Kobilka, B.K. Structure of the delta-opioid receptor bound to naltrindole. Nature 2012, 485, 400–404.
- Chavkin, C.; Goldstein, A. Specific receptor for the opioid peptide dynorphin: Structure--activity relationships. Proc. Natl. Acad. Sci. USA 1981, 78, 6543–6547.
- Portoghese, P.S.; Sultana, M.; Takemori, A.E. Design of peptidomimetic delta opioid receptor antagonists using the message-address concept. J. Med. Chem. 1990, 33, 1714–1720.
- Schiller, P.W.; Nguyen, T.M.; Chung, N.N.; Lemieux, C. Dermorphin analogues carrying an increased positive net charge in their “message” domain display extremely high mu opioid receptor selectivity. J. Med. Chem. 1989, 32, 698–703.
- Borics, A.; Toth, G. Structural comparison of mu-opioid receptor selective peptides confirmed four parameters of bioactivity. J. Mol. Graph Model 2010, 28, 495–505.
- Lasota, A.; Frączak, O.; Muchowska, A.; Nowakowski, M.; Maciejczyk, M.; Ejchart, A.; Olma, A. Synthesis, Biological Activity, and NMR-Based Structural Studies of Deltorphin I Analogs Modified in Message Domain with a New α, α-Disubstituted Glycines. Chem. Biol. Drug Design 2016, 87, 824–832.
This entry is offline, you can click here to edit this entry!
