Wood used outdoor is subjected to different sources of degradation and should be protected properly. In this study, acrylic resins were added to a wood impregnation system using amine oxides and propiconazole, an organic fungicide, to create a two-part wood protection preservation treatment. Since amine oxides can diffuse readily into wood, this treatment protected both the surface and inner structure of the treated wood following a simple dipping. Many aspects of the treatment were studied: the adhesion of the acrylic coatings, their permeability to water, and the impregnation depth of the propiconazole. In each case, a particular attention was accorded to the interactions between the resins and the impregnation system. Adhesion and permeability tests were coupled with an artificial aging process simulating severely wet conditions. Amine oxides reduced the adhesion of the coatings, but did not impair their aging properties. Because of their hydrophilic nature, they also increased the permeability to liquid water, although they did not affect the air moisture permeability. The penetration of the propiconazole, estimated with a dye, decreased with the resin. Overall, the two parts of the treatment lightly impaired each other, but the practical aspect of this treatment may overcome these disadvantages.
- acrylic coating
- amine oxides
- propiconazole
- wood protection
- adhesion
- permeability
- impregnation
- artificial aging
- white pine
- white spruce
1. Introduction
The use of wood in buildings should be promoted when it is possible, as it is a bio-based material with great properties and aesthetics. Other materials, such as steel and concrete, are sometimes preferred to wood as they are considered more durable. It is however possible to efficiently protect wood from decay and damages with simple treatments.
Wood protection could mainly be separated in two broad and complementary categories: impregnation treatments and coatings. Impregnation treatments are used to penetrate wood cavities with hydrophobic and/or biocidal compounds and improve its durability against insects, decay fungi, molds and dimensional changes [1][2][3][4][5][6]. Some reactive chemicals, like acetic anhydride and formaldehyde, can also be used to modify the chemical nature of the cell walls and improve their properties [7][8]. Wood impregnation usually relies on pressure and vacuum methods to allow for a rapid and deep penetration of the treatments [9][10]. Coatings can be very diverse, from penetrating oils to film-forming alkyds and acrylics [11]. They can prevent the weathering from abiotic elements (wind, sand, rain, etc), decrease the exchanges of moisture, and block the UV rays from the sun with pigments and UV absorbers [12][13][14][15]. The natural look of the wood surface can be preserved with clear coatings, or altered to hidden with increasing amount of pigments.
An aqueous impregnation treatment was recently developed to allow for the impregnation of wood through diffusion after a simple dipping, preventing the need for pressure and vacuum [16]. It uses the abilities of tertiary amine oxides to diffuse into the wood and solubilize organic compounds, like biocides, to protect wood from biodegradation and dimensional changes [17][18]. In a previous paper, we showed that this treatment could nearly inhibit the fungal degradation by Rhodonia placenta and decrease the dimensional changes in eastern white pine (Pinus strobus L) and white spruce (Picea glauca Moench (Voss)) by 29% and 24%, respectively, while barely increasing their density [19]. In another paper, we determined that the treatment did improve the penetration of the fungicides, but only longitudinally. Meanwhile, the antiseptic amine oxides could penetrate perpendicularly to the grain, granting fungal protection below the surface. We also found that the treatment allowed to impregnate enough fungicide to respect the EN standards, even after 2 weeks of leaching by immersion [20].
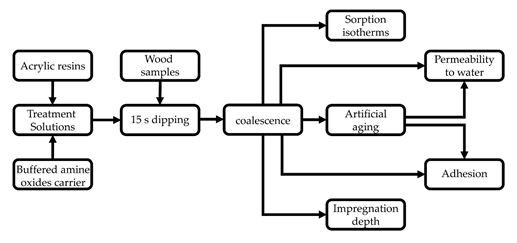
In this paper, we brought the treatment one step further by adding acrylic resins to its composition. It allowed for both the impregnation and the coating of wood in a single step. After characterizing the treatment solutions and the dry films, we tested the adhesion of the films, their permeability to water and air moisture, and the impregnation depth of an indigo blue dye (Fig. 1). The adhesion and water permeability tests were combined with artificial aging. The main results are summarized in the next section and discussed in further details in the paper.
Figure 1. Experimental procedure of the study
2. Main results
2.1. Properties of the treatments
The treatment solutions were prepared using a factorial design with acrylic resins and amine oxides (AO) conditions as the factors. They would contain no resin (R0) or one of three commercial resins (R1, R2, R3), and either no amine (AO0), only dimethyldodecylamine oxide (DDAO)(AO1), or a mix of DDAO and dimethylhexadecalmine oxide (DHAO)(AO2).
We found that both the amine oxides and the acrylic resins increased the viscosity of the treatment solutions. Consequently, the dry films of the treatments containing amine oxides were also thicker. The amine oxides usually led to a faster drying time and a lower Tg, particularly for AO2.
Table 1. Properties of the treatment solutions (at 65 oC) and of the dry coatings.
|
Treatment |
Viscosity (cP) |
Film thickness (μm) |
Drying time (s) |
Theorical Tg* (oC) |
Experimental Tg (oC) |
Particle size* (μm) |
|
|
Pine |
Spruce |
||||||
|
R0-AO0 |
0.63 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
R0-AO1 |
0.77 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
R0-AO2 |
6.47 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
R1-AO0 |
56 |
65.5 |
60 |
461 |
15 |
11.01 |
0.10 |
|
R1-AO1 |
66 |
62.5 |
68.5 |
431 |
15 |
6.90 |
0.10 |
|
R1-AO2 |
91 |
88.5 |
62.5 |
375 |
15 |
5.06 |
0.10 |
|
R2-AO0 |
32 |
40.5 |
49.5 |
165 |
20 |
14.69 |
0.23 |
|
R2-AO1 |
66 |
53.5 |
47.5 |
114 |
20 |
11.34 |
0.23 |
|
R2-AO2 |
164 |
62.0 |
83.5 |
2344 |
20 |
8.96 |
0.23 |
|
R3-AO0 |
27 |
55.5 |
55.3 |
130 |
14 |
11.55 |
0.30 |
|
R3-AO1 |
46 |
62.0 |
63.5 |
144 |
14 |
15.18 |
0.30 |
|
R3-AO2 |
66 |
62.5 |
69 |
161 |
14 |
11.07 |
0.30 |
*These properties are provided by the supplier of the acrylic resins
2.2. Permeability to water
The permeability to water of the treatments was studied by comparing the mass of water absorbed by treated and untreated samples after 30 minutes of soaking [21]. The water repellent efficiency (WRE) was calculated with eq. 1:
WRE (%) = 100 x ((Mua - Mub) - (Mta - Mtb))/(Mua - Mub)
where Mua and Mub represent the mass (in grams) of untreated samples after and before soaking, and Mta and Mtb the mass (in grams) of treated samples after and before soaking, respectively. The samples for this test were either unaged or aged for 14 cycles (Table 2).
Table 2. Conditions of one cycle of artificial aging.
|
Conditions |
Temperature (oC) |
Relative humidity |
Duration |
|
Rainy night |
5 oC |
98% + rain |
10 h |
|
Cold night |
5 oC |
98% |
22 h |
|
Winter |
-15 oC |
N/A |
8 h |
|
Dry day |
50 oC |
20% |
8 h |
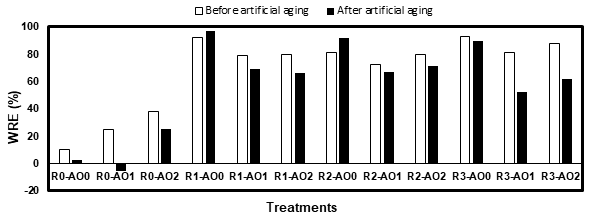
The results showed that the amine oxides reduced the permeability to water of uncoated samples (R0-AO1 and R0-AO2), but increased the permeability of the coated ones. It was attributed to the amine oxides being less hydrophilic than the wood cells, but more than the acrylic resins. In both scenarios, the treatments with DHAO (AO2) were less hydrophilic. All three resins reduced significantly the permeability to water of white spruce before aging. They however did not perform well after aging as the samples were cracked, which greatly increased the absorption of water. This cracking was attributed to the freezing of trapped water into the wood during the aging cycles.
Figure 2. Water repellent efficiency (WRE) of the white spruce before and after artificial aging.
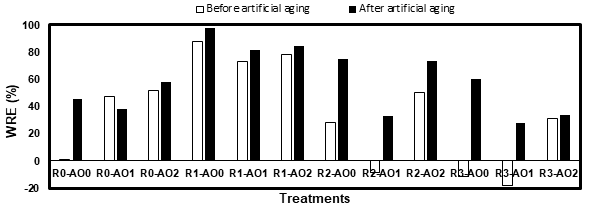
Figure 3. Water repellent efficiency (WRE) of the white pine before and after artificial aging.
In the case of white pine, R2 and R3 did not perform well before aging, which would be caused by an insufficient protection of the latewood (Fig. 4). The WRE of these samples however increased by a large margin after artificial aging, as the resins still managed to prevent these samples from cracking during the aging process.
Figure 4. Optical microscopy of the transverse plane of white spruce and white pine treated with R1-AO1 and R2-AO1. The arrows indicate the presence of resin at the surface of the latewood.
2.3. Permeability to moisture
The permeability to moisture was investigated with sorption isotherms. It was observed that, for uncoated samples, the absorption of moisture was greater and faster than for untreated samples. It was attributed to the ability of amine oxides to make hydrogen bonds with water, which added many sites into the wood for water adsorption [22]. The resins did not seem to reduce the absorption of water. Conversely, their EMC was much higher than the uncoated samples, suggesting that the coatings absorbed a lot of moisture. On the other hand, they did slow down the uptake of moisture.
Figure 5. Equilibrium moisture content (EMC) of the samples (left) and time elapsed after reaching the equilibrium (right) at the different steps during the sorption isotherms in white pine.
Figure 6. Equilibrium moisture content (EMC) of the samples (left) and time elapsed after reaching the equilibrium (right) at the different steps during the sorption isotherms in white spruce.
2.4. Adhesion
The adhesion of the acrylic resins was tested with pull-off tests using 20 mm dollies glued to their surface. The samples used for this test were artificially aged for 0, 1, 7, or 14 cycles (Table 2).
Although the adhesion was quite low for all the treatments, the fracture occurred at the wood/coating interface for all the treatments, except some aged R2-AO1 pine samples and some unaged R2-AO2 spruce samples. It was found that the amine oxides decreased the adhesion of the coatings, particularly for AO2, which would result from a lower Tg. The artificial aging improved the adhesion of the coatings after the first cycle, as water soluble compounds were lost. The adhesion remained higher up to the seventh cycles, but then decreased as the bounds between the wood and the coatings began to break.
Figure 7. Tensile strength required to pull-off the dollies from white pine (left) and white spruce (right)
2.5. Impregnation depth
The impregnation depth of organic fungicides was estimated with an indigo blue dye. To do this, cubic samples were treated with solutions containing the dye. These samples were then sawn open and the penetration of the indigo was measured with an optical microscope.
The penetration of the indigo dye was only observed longitudinally. In the case of the white pine, the less viscous solutions, primarily those without any resin, penetrated slightly into the earlywood and deeper into the more permeable latewood. However, as a result of the greater capillary forces, the white spruce samples, as well as the pine samples treated with solutions containing a resin, were only impregnated in the earlywood. The impregnation depth was usually inversely proportional to the viscosity of the treatment solution.
Figure 9. Impregnation depth of the indigo dye in the white pine (earlywood and latewood) and the earlywood of the white spruce.
3. Conclusion
After treating wood samples with solutions combining a buffered amine oxide impregnation system and acrylic resins, the following statements could be made:
-
The combination of the two parts of the treatments successfully allowed both the coating and the impregnation of wood in a single step.
-
The two parts of the treatments affected the properties of the treatment solutions and of the dry films.
-
The impregnation system usually led to a loss of adhesion of the coatings.
-
The impregnation system slightly increased the permeability to water of the coatings.
-
The impregnation system did not impair the impermeability to moisture of the coatings.
-
The acrylic resins decreased the impregnation depth as a result of the higher viscosity of the solutions.
Accordingly, it was established that the practical side of the addition of an acrylic resin to an impregnation treatment would be paid with some performances. While much work should be done to optimize the treatment and lessen this side-effect, it can still be useful in specific situations, like sidings protected from the rain, which are not affected too much by the adhesion and water permeability of the coating. Adding more layers of coating could also help to improve the overall performances and durability of the coating.
This entry is adapted from the peer-reviewed paper 10.3390/coatings10040366
References
- Wang, C.; Piao, C. From Hydrophilicity to Hydrophobicity: A Critical Review - Part II: Hydrophobic Conversion. Wood Fiber 2010, 42, 490–510.
- Kocaefe, D.; Huang, X.; Kocaefe, Y. Dimensional Stabilization of Wood. Curr. For. Rep. 2015, 1, 151–161.
- Reinprecht, L. Wood Deterioration, Protection, and Maintenance, 1st ed.; John Wileys & Sons: Chichester, UK, 2016; ISBN 9781119106531.
- Schultz, T.; Nicholas, D. A Brief Overview of Non-Arsenical Wood Preservative Systems. In Wood Deterioration and Preservation: Advances in Our Changing World; Goodell, B., Nicholas, D.D., Schultz, T.P., Eds.; American Chemical Society: Washington, DC, USA, 2003; Volume 845, pp. 420–432. ISBN 9780841237971.
- Laks, P.E. Wood Preservative Fungicides and the American Wood Preservers’ Association Use Category System. In Development of Commercial Wood Preservatives; Schultz, T.P., Militz, H., Freeman, M.H., Goodell, B., Nicholas, D.D., Eds.; American Chemical Society: Washington, DC, USA, 2008; Volume 982, pp. 228–240. ISBN 978-0-8412-3951-7.
- Ross, A.S. Organic Preservative Systems for the Protection of Wood Windows and Doors. In Development of Commercial Wood Preservatives; Schultz, T.P., Militz, H., Freeman, M.H., Goodell, B., Nicholas, D.D., Eds.; American Chemical Society: Washington, DC, USA, 2008; Volume 982, pp. 470–479. ISBN 978-0-8412-3951-7.
- Kumar, S. Chemical Modification Of Wood. Soc. Wood Sci. Technol. 1994, 26, 270–280.
- Yuan, J.; Hu, Y.; Li, L.; Cheng, F. The Mechanical Strength Change of Wood Modified with DMDHEU. BioResources 2013, 8, 1076–1088.
- Leightley, L.E. Protection of Wood Using Combinations of Biocides. In Wood Deterioration and Preservation: Advances in Our Changing World; Goodell, B., Nicholas, D.D., Schultz, T.P., Eds.; American Chemical Society: Washington, DC, USA, 2003; Volume 845, pp. 420–432. ISBN 9780841237971.
- Freeman, M.H. Wood Preservative Formulation Development and Systems: Organic and Inorganic Based Systems. In Development of Commercial Wood Preservatives; Schultz, T.P., Militz, H., Freeman, M.H., Goodell, B., Nicholas, D.D., Eds.; American Chemical Society: Washington, DC, USA, 2008; pp. 408–426. ISBN 978-0-8412-3951-7.
- Bulian, F.; Graystone, J.A. Industrial Wood Coatings, 1st ed.; Elsevier: Oxford, UK, 2009; ISBN 9780444528407.
- de Meijer, M. Review on the Durability of Exterior Wood Coatings with Reduced VOC-Content. Prog. Org. Coat. 2001, 43, 217–225.
- Teng, T.-J.; Mat Arip, M.N.; Sudesh, K.; Nemoikina, A.; Jalaludin, Z.; Ng, E.-P.; Lee, H.-L. Conventional Technology and Nanotechnology in Wood Preservation: A Review. BioResources 2018, 13, 9220–9252.
- Müller, B.; Poth, U. Coatings Formulations, 2nd ed.; Vincentz Network: Hanover, Germany, 2011; ISBN 978-3-86630-891-6.
- Miller, E.R.; Boxall, J. Water-Borne Paints for Exterior Wood. Pigment Resin Technol. 1984, 13, 15.
- Shen, S.; Walker, L.E. Methods for Enhancing Penetration of Wood Preservatives. Patent WO2000059696A2, 2001.
- Tseng, C.-I.; Walker, L.E. Azole/Amine Oxide Wood Preservatives. Patent WO2000071314A1, 2000.
- Ross, A.S.; Cutler, K.A. Method of Employing Enhanced Penetration of Wood Preservatives to Protect Wood and a Related Solution. U.S. Patent 20120258248A1, 2014.
- Pepin, S.; Blanchet, P.; Landry, V. Characterization of the Diffusion of Organic Fungicides with Amine Oxides in White Pine and White Spruce. BioResources 2020, 15, 1026–1049.
- epin, S.; Blanchet, P.; Landry, V. Performances of White Pine and White Spruce Treated with Organic Fungicides Using an Aqueous Buffered Amine Oxide Preservation System. BioResources 2019, 14, 264–288.
- ASTM D5401-03. Standard Test Method for Evaluating Clear Water Repellent Coatings on Wood; ASTM International: West Conshohocken, PA, USA, 2014.
- Kocherbitov, V.; Veryazov, V.; Söderman, O. Hydration of Trimethylamine-N-Oxide and of Dimethyldodecylamine-N-Oxide: An Ab Initio Study. J. Mol. Struct. THEOCHEM 2007, 808, 111–118.