2. Oxidative Stress and NDs
Oxidative stress, the result of an imbalance in the relative abundance of reactive ROS and antioxidants, can create a detrimental state that leads to cellular damage and dysfunction. Increased oxidative stress is able to damage cell membranes, alter protein structure and function, and cause DNA damage [
25,
26]. Therefore, maintenance of redox homeostasis is essential for cell biological function. As mentioned earlier, the CNS is particularly sensitive to oxidative stress due to its high metabolic rate, relative antioxidant scarcity, and unique structural features [
27,
28]. In addition, due to the presence of high levels of metal ions and polyunsaturated fatty acids, neuronal cells are more prone to oxidative stress, leading to cell damage and a series of NDs-related events through mitochondrial dysfunction, inflammation, and neuronal death [
28,
29,
30]. In fact, an oxidative stress-induced imbalance in redox homeostasis is still a central component of the pathogenesis of several NDs, such as AD, PD, and MS. The common features among these NDs are ineffective antioxidant defense systems, imbalances of redox homeostasis, mitochondrial dysfunction, neuroinflammation, neuronal loss and degeneration (
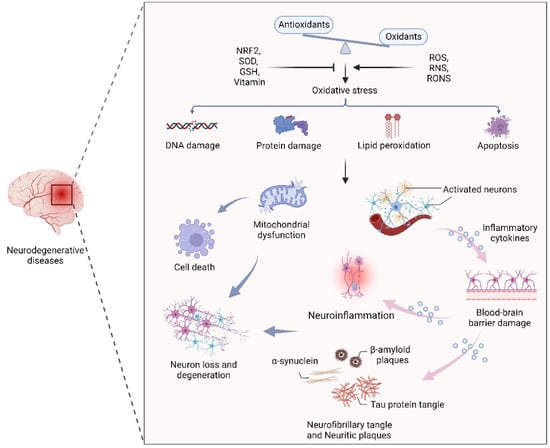
Figure 1).
Figure 1. The effect of oxidative stress in neurodegenerative diseases. An oxidant/antioxidant imbalance leads to oxidative stress, which causes DNA and protein damage, lipid peroxidation, and apoptosis. Dysfunctional mitochondria and activated neurons secrete inflammatory cytokines that cross the blood–brain barrier, leading to inflammation, α-synuclein, β-aggregation, and neuronal plaque accumulation in neurons, leading to neuron loss and degeneration.
Oxidative stress and disruption of cerebral redox homeostasis frequently occur in human NDs. For instance, in the pathology of AD, amyloid β (Aβ) and tau protein aggregates can interact with metal ions and maintain normal cellular signaling [
31,
32]. Furthermore, previous studies have shown that the high levels of zinc in the neocortical and hippocampal regions of AD patients suggest the vital role of zinc in the maintenance of redox homeostasis in the affected brain regions [
33,
34]. Notably, accumulated Aβ-induced oxidative stress can inhibit the activity of complex IV, leading to ATP depletion and mitochondrial dysfunction [
35,
36]. It has been demonstrated that the abnormal aggregation of α-synuclein (α-syn), mitochondrial dysfunction, and excessive oxidative stress are closely related to dopaminergic neuron death during PD progression [
37,
38,
39]. As the main pathogenic factor of HD, soluble and aggregated mutant Htt (mHtt) protein with cytotoxicity induces apoptosis through oxidative stress, resulting in continuous degeneration of neurons [
40,
41,
42]. Interestingly, in patients with NDs, oxidative stress biomarkers such as malondialdehyde and 8-hydroxyguanosine are elevated, and the gene superoxide dismutase 1 (SOD1), which plays an important role in oxidative stress defense mechanisms, is also frequently mutated [
43,
44].
Despite the advanced understanding of the mechanisms described above, a wide gap remains between this knowledge and the availability of effective therapies. Given that the imbalance of redox homeostasis is one of the key factors in the pathogenesis of NDs, numerous studies have been conducted on the treatment of NDs using various types of antioxidants (Table 1). Overall, most of the clinical trial results of NDs have shown favorable therapeutic effects, especially the alterations of pathological markers and improvements in neurological function, suggesting that antioxidants have great therapeutic potential for NDs. However, more efforts are required to explore novel therapeutic strategies and approaches to achieve broad therapeutic applicability and functional recovery of the nervous system.
Table 1. Antioxidants with therapeutic effects on neurodegenerative diseases.
| Antioxidants |
Therapeutic
Target |
Mechanism |
Reference |
| Luteolin |
PD |
Increased dopamine absorption |
[45] |
| Selenium |
AD |
Degradation of Aβ plaques |
[46,47] |
| Curcumin |
PD |
NRF2 activation |
[48] |
| α-Tocopherol |
AD |
Aβ plaque degradation |
[49] |
| Quercetin |
AD, PD |
Hydroxyl radical scavenging |
[50] |
| Ginsenosides |
AD |
Inhibition of Aβ aggregation |
[51] |
| PLGA NPs |
AD, PD, MS |
Protection against oxidative stress |
[52] |
Macrophage-derived
exosomes |
PD |
Protection against oxidative stress and inflammation |
[53] |
| Coenzyme Q10 |
AD |
Reduction of oxidative stress and senile plaques |
[54] |
| Ferulic acid |
AD |
Inhibition of neuronal oxidative stress |
[55] |
3. Gut Microbiota, Oxidative Stress, and Neurodegeneration
The human gastrointestinal tract is the largest immune organ and harbors complex and dynamic microbiota [
56,
57]. Gut microbiota stability can be impacted by several variables, including genetics, lifestyle, nutrition, medications, illness, and age, which in turn have a significant impact on the regulation of metabolism, homeostasis, immunological response, and other processes [
58,
59]. Therefore, an imbalance in the representation of the gut microbiota may contribute to various diseases, from inflammatory bowel disease to obesity and diabetes, as well as several common NDs, such as AD, PD, and MS (
Table 2). In addition, growing numbers of studies have demonstrated that the gut microbiota alters the oxidative/antioxidant balance in the CNS and causes neurodegeneration [
60,
61,
62,
63].
Table 2. Alterations in the gut microbiota composition in various neurodegenerative diseases.
Neurodegenerative
Disease |
Experimental
Subject |
Gut Microbiota |
Reference |
| AD |
Fecal samples from AD |
Firmicutes, Bifidobacterium ↓
Bacteroidetes ↑ |
[64] |
| |
Symptomatic
Tg2576 mice |
Firmicutes, Bacteroidetes, Lactobacillus ↑ |
[65] |
| |
Fecal samples from
AD patients |
Ruminococcacea ↑ Lachnospirace ↓ |
[66] |
| |
Male patients with AD |
Bacteroidetes,
Blautia ↑
Firmicutes, Bifidobacterium ↓ |
[67] |
| |
Amyloid-positive
patients |
Escherichia,
Shigella ↑
Eubacterium
rectale ↓ |
[68] |
| PD |
Patients with PD |
Enterobacteriaceae, Serratia ↑
Blautia, Coprococcus, Lachnospiraceae ↓ |
[69] |
| |
16S microbiome
datasets |
Akkermansia,
Lactobacillus, Bifidobacterium ↑ Faecalibacterium, Lachnospiraceae ↓ |
[70] |
| |
Patients with PD |
Butyricicoccus,
Clostridium ↑ Shigella, Lactobacillus ↓ |
[71] |
| MS |
Patients with MS |
Caproic acid, producers ↑
Butyric acid, producers ↓ |
[72] |
| |
Patients with MS |
Patescibacteria ↑ Lachnospiraceae, Ruminococcaceae ↓ |
[73] |
3.1. Gut–Brain Axis under Physiological Conditions
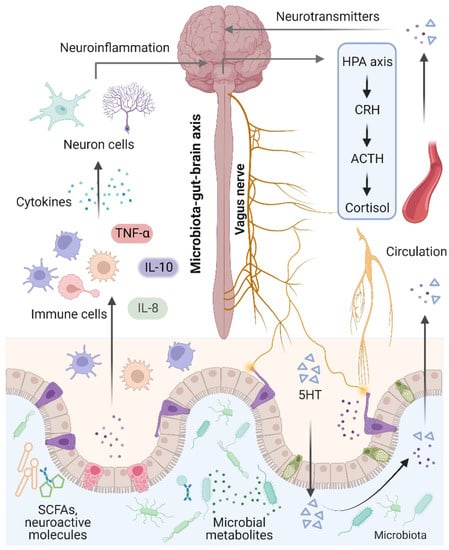
The brain and the gut microbiota are strictly intertwined and communicate in a variety of ways, including the production of bacterial metabolites, neurotransmitters, and cytokines [
74] (
Figure 2). Notably, the term “microbiota–gut–brain axis” refers to an interaction between the brain and the gut microbiota that involves four major routes of communication [
75,
76]. The first route of communication involves the vagus nerve, which connects the enteric nerve system and the brain stem. Recent research indicates that the gut microbiota influences host behaviors such as anxiety, feeding, and depression by activating vagal neurons and altering neurotransmitters such as γ-aminobutyric acid (GABA) and oxytocin in the brain [
77,
78]. The second important mode that directly or indirectly affects brain activity involves serotonin, which is mainly produced by gut enterochromaffin cells and modulates a variety of physiological processes. Interestingly, increased levels of serotonin and serotonin precursors alleviated depression in a mouse model of depression after treatment with the probiotic Bifidobacterium [
79]. Thirdly, the gut microbiota plays an essential role in microglial activation and neuroinflammation. For instance, Luck and colleagues demonstrated that germ-free mice carry more immature microglia than conventional mice, and
Bifidobacterium spp. can activate microglia through transcriptional activation [
80]. In addition, alterations in microglial function were also observed in NDs and other behaviors, suggesting that the gut microbiota mediates effects on NDs through microglia [
81]. Notably, the gut microbiota plays a vital role in energy harvest and neuroinflammation, and alterations in the gut–brain vagal pathway may promote obesity. It has been demonstrated that a diet-induced shift in the gut microbiome may disrupt vagal gut–brain communication resulting in microglia activation, increased gut inflammation, and body fat accumulation [
82,
83]. Finally, the gut microbiota communicates by transferring chemical signals directly to the brain. A previous study indicated that short-chain fatty acids (SCFAs) derived from the fermentation of the gut microbiota had been shown to modulate neuroplasticity in the CNS and improve depressive behavior in mice [
62].
Figure 2. Microbiota–gut–brain axis. The brain and gut communicate through neural, metabolic, endocrine, and immunological pathways. The brain influences gut health through the vagus nerve, the hypothalamic–pituitary–adrenal (HPA) axis, and systemic circulation. Signals from the gut, including short-chain fatty acids (SCFAs), neurotransmitters, and amino acids, modulate brain function via neuronal cells, the immune system, and endocrine mechanisms.
3.2. Gut Microbiota-Mediated Oxidative Stress and Neurodegeneration
There are four main symbiotic bacteria that are parasitic in the human gut, namely Actinobacteria, Bacteroidetes, Proteobacteria, and Firmicutes. Among them, Firmicutes accounts for the largest proportion, including Streptococcus, Lactobacillus, and Mycoplasma [
81]. Recent studies have found that, in the presence of the microbiota, the intestinal epithelium cells produce physiological levels of oxidative stress that affect the composition and function of the gut microbiota. Such alterations in gut microbiota increase the alterations of biomacromolecules reaching the systemic circulation and CNS by directly affecting the permeability of the intestine [
84]. Indeed, the gut microbiota can alter cellular oxidative stress status by regulating mitochondrial activity [
85]. In addition, gut
Lactobacilli,
Bifidobacterium, and
Streptococcus can produce nitric oxide (NO) in various ways in the gut [
86,
87]. It is now generally accepted that NO in nanomolar concentrations is neuroprotective, whereas higher concentrations of NO may result in oxidative stress, which is closely related to axonal degeneration, neuroinflammation, and NDs [
81]. In addition, certain pathogenic bacteria such as
Salmonella and
Escherichia coli are able to produce hydrogen sulfide (H
2S) in the gut by degrading sulfur-containing amino acids. Furthermore, increased levels of H
2S alter various host metabolic activities, such as increased lactate, decreased oxygen consumption, decreased ATP production, and elevated levels of proinflammatory compounds, which have been linked to neuroinflammation [
88,
89,
90]. The role of gut microbiota in neurodegeneration is shown in
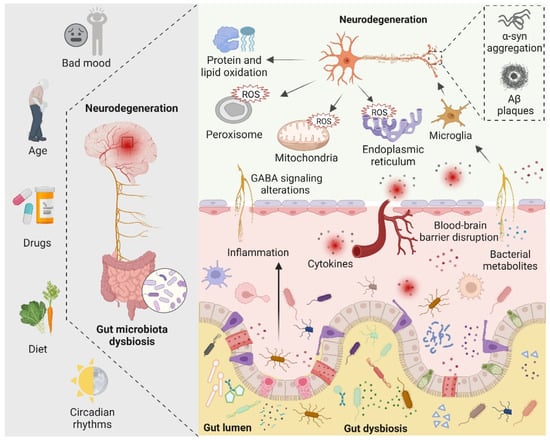
Figure 3.
Figure 3. The role of the gut microbiota in neurodegeneration is depicted schematically. Bad mood, increasing age, drugs, dietary changes, and circadian rhythms can disrupt gut microbiota homeostasis. When gut dysbiosis occurs, beneficial bacteria in the gut are transformed into pathogenic bacteria, producing a large number of harmful metabolites and proinflammatory molecules, resulting in increased blood–brain barrier permeability and peripheral inflammatory responses, thereby aggravating oxidative stress in the brain. At the same time, dysbiosis can induce bad mood. Increased levels of ROS in neuronal mitochondria, endoplasmic reticulum, and peroxisomes, increased protein and lipid oxidation, and accumulation of neurotoxic proteins lead to neurodegeneration.
3.2.1. Alzheimer’s Disease
AD is the most common ND worldwide with an insidious onset and progressive development [
4,
91]. It is characterized by progressive impairment of cognition and episodic memory, culminating in the development of dementia [
92]. Specific histopathological hallmarks in the brain associated with AD include Aβ plaques, neurofibrillary tangles (NFTs), hyperphosphorylation of tau proteins (tau tangles), and neuronal loss [
93,
94]. Oxidative stress has been suggested to play an essential role in AD etiology prior to plaque formation, leading to mitochondrial dysfunction in neurons and synapses, as well as Aβ protein production [
95,
96]. Previous studies have shown that oxidative stress plays a pivotal role in the development of AD. For instance, it has been demonstrated that aggregated Aβ protein stimulates microglia to produce ROS through positive feedback on Aβ plaque deposition [
91]. In addition, tau protein aggregation in neurons leads to reduced NADH-ubiquitin reductase activity, leading to oxidative stress and mitochondrial dysfunction [
97]. Interestingly, ROS can affect the activity of stress kinases, such as the phosphorylation-c-Jun N-terminal kinase 1 (p-JNK) pathway, which is associated with neuronal cell death due to tau hyperphosphorylation and accumulation of Aβ [
98]. There is ample evidence that the oxidation of nucleic acid species in the AD brain is dominated by the mitochondrial genome, and lipid peroxidation results in the production of certain cytotoxic agents, such as 4-hydroxyalkenals [
99,
100,
101].
Recent studies have shown that the gut microbiota plays a significant role in the pathogenesis of AD [
102]. Dysregulation of the gut microbiota leads to oxidative stress, inflammation, disruption of the blood–brain barrier, activation of the immune system, neurofibrillary tangles, and Aβ plaques followed by neurodegeneration [
21,
103]. There are numerous bacteria in the human gut that play a vital role in the etiology of AD, including
Staphylococcus aureus,
Escherichia coli,
Salmonella,
Mycobacterium,
Klebsiella pneumoniae, and
Streptococcus, which promote the production and aggregation of the Aβ protein in the enteric nervous system [
104,
105]. Interestingly, in the APP
SWE/PS1
ΔE9 transgenic mouse model of AD chronically treated with broad-spectrum combination antibiotics, the gut microbiome of transgenic mice shifted toward proinflammatory bacteria, with a decrease in amyloid plaque deposition and neuroinflammation [
66,
106]. Additionally, microbial amyloid protein, produced by coccus-shaped bacteria, is able to activate the innate immune system and triggers responses by Toll-like receptors (TLRs) and cluster of differentiation 14 (CD14), resulting in inadequate recognition of misfolded Aβ and decreased Aβ clearance, followed by the production of cytokines leading to intestinal disturbances [
107]. Notably, age-related reductions in gut microbial diversity are also implicated in AD. It has been demonstrated that with growing age, there is an increase in Proteobacteria and a decrease in Bifidobacterium spp., which results in interference in lipid metabolism and a failure to maintain hippocampal plasticity as well as memory functions [
108,
109].
Another possible connecting link between the gut microbiota and microbiota-mediated cerebral amyloid accumulation involves a cross-seeding mechanism of microbial amyloid (i.e., promoting misfolded aggregation of amyloid from one protein to another) in a manner similar to the reproduction of prions [
107,
110,
111]. Notably, distinct amyloid conformations interact with cellular targets to produce various toxicities, which may explain the different AD phenotypes [
112]. Given the multiple roles of gut microbiota dysbiosis in the pathogenesis of AD, modulation of AD through dietary and gut microbiota interventions may be potential therapeutic strategies, which will be discussed in detail later.
3.2.2. Parkinson’s Disease
PD, the second most common ND after AD, is a long-term neurological disorder that causes both motor and non-motor symptoms [
113,
114,
115]. Motor symptoms include resting tremors, akinesia, muscular rigidity, postural instability, and gait abnormalities [
116,
117,
118]. Non-motor symptoms include anxiety, depression, autonomic dysfunction, cognitive decline, and sleep disturbances [
119,
120]. The hallmarks of PD are loss of dopaminergic (DA) neurons and abnormal accumulation of α-syn within the cytoplasm of nerve cells called Levy bodies [
121,
122,
123,
124]. Notably, oxidative stress, mitochondrial dysfunction, dopamine metabolism, abnormal protein aggregation, and the gut microbiota are associated with the pathogenesis of PD [
125,
126,
127]. As one of the main pathogenic factors of PD, oxidative stress has been linked to α-syn protein aggregation and degeneration in DA neurons [
98,
121,
128]. For instance, analysis of the postmortem brain tissue in PD showed that oxidative stress degenerates DA neurons, reduces the levels of glutathione (GSH), increases the levels of oxidative stress markers, and stimulates lipid, DNA, and RNA oxidation [
129,
130]. Additionally, Tong and colleagues have demonstrated that oxidative stress in DA neurons can activate the p38 mitogen-activated protein kinase pathway, ultimately leading to neuronal apoptosis [
131]. Interestingly, in the 6-hydroxydopamine-induced PD model in mice, Antrodia camphorata polysaccharide reduced ROS by increasing the expression and activity of antioxidant enzymes, ultimately attenuating the damage of DA neurons in the substantia nigra and improving motor performance [
132].
Notably, PD patients often present with gastrointestinal dysfunction, which suggests that the imbalance of the gut microbiota is one of the causes of triggering or aggravating PD [
133,
134]. Indeed, gut inflammation, early accumulation of α-syn, increased intestinal permeability, and constipation problems are common in PD patients, again demonstrating the critical role of the gut microbiota in PD [
81,
135]. It has been demonstrated that the disruption of gut microbiota leads to oxidative stress through overstimulation of the immune system, which in turn activates intestinal neurons and intestinal glia cells, leading to increased misfolding and accumulation of α-syn in the CNS [
136,
137]. In addition, coming to the role of the gut microbiota, toxins and microbial products produced by certain pathogenic bacteria are able to cause mitochondrial dysfunction in intestinal cells and the CNS, which is directly associated with PD pathogenesis [
138]. In line with this, it is proposed that the pathogenic bacterium
E. coli can produce an amyloid protein called curli, which promotes the accumulation of α-syn protein in the brain and causes motor defects in mice [
139]. Conversely, when treated with gut-restricted amyloid inhibitors, these mice showed significant improvements in constipation and motor function, suggesting the role of the gut microbiota in the etiology of PD symptoms [
140]. Interestingly, when the gut microbiota from PD patients was transplanted into a germ-free α-syn overexpressed mouse model, a similar pattern of physical injury to PD patients was observed, suggesting the vital role of the gut microbiota in PD. As discussed above, decreased production of hydrogen (H
2) by the gut microbiota has been proposed as one of the essential factors in PD [
141]. According to a recent study, 50% H
2 saturated water was successful in preventing nigrostriatal degeneration in PD rats and reducing the oxidative stress markers in the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine (MPTP) mouse model [
142]. Taken together, these observations suggest a critical role for gut microbiota in PD, and intervention through gut microbiota is expected to provide promising strategies for the prevention and treatment of PD.
3.2.3. Multiple Sclerosis
MS is an immune-mediated chronic inflammatory and central nervous system demyelinating disease with a complex and unclear pathogenesis [
143]. It has been established that the pathogenesis of MS involves both genetic and environmental factors. The most extensively accepted hypothesis is that autoreactive B and T cells cause axonal and myelin damage, as well as neurodegeneration [
144,
145]. The major neuropathological hallmarks of MS pathology are inflammation and degeneration of both white matter and gray matter [
146]. However, the development of MS may be influenced by a combination of internal and external factors, ultimately leading to immune dysregulation.
Growing evidence suggests that the imbalance of redox homeostasis plays a vital role in the pathogenesis of MS. For instance, it has been demonstrated that the excessive generation of ROS, mitochondrial dysfunction, and impairment of antioxidant defense systems play important roles in the pathogenesis of MS [
147]. Notably, ROS has been shown to be a mediator of axonal injury and demyelination in both MS patients and animal models of MS. In addition, oxidative stress mediates mitochondrial dysfunction in MS patients and leads to CNS energy failure in MS-susceptible individuals [
148,
149]. Furthermore, recent studies have demonstrated that the gut microbiota has a significant impact on MS and can be influenced by external factors [
150]. For instance, Cosorich et al. have demonstrated that T helper 17 (TH17) cells, key players in MS, originate in the gut and that increased TH17 cell frequency is associated with specific alterations of the gut microbiota in MS patients [
151]. Interestingly, transplantation of the MS microbiota in a mouse model resulted in an increased incidence of autoimmune encephalomyelitis, leading to an exacerbation of MS symptoms [
152,
153]. Additionally, diets have been shown to affect the balance of the gut microbiota and indirectly influence the development of MS [
154]. Moreover, dietary studies in MS patients suggest that dietary interventions supplemented with vitamin D in a low-calorie diet have a positive effect on alleviating chronic inflammatory symptoms in MS [
155]. Recently, intermittent fasting was introduced into the treatment of MS due to its availability of abundant gut microbiota as well as the secretion of glutathione and leptin [
156]. All these studies have shown that modification of the gut microbiota can be considered a promising therapeutic strategy for MS.