Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
External cavity quantum cascade lasers (ECQCLs) in the mid-infrared band have a series of unique spectral properties, which can be widely used in spectroscopy, gas detection, protein detection, medical diagnosis, free space optical communication, and so on, especially wide tuning range, the tuning range up to hundreds of wavenumbers; therefore, ECQCLs show great applications potential in many fields.
- detection
- ECQCL
- QCL
- tunable
1. Introduction
The operating wavelength of III-V PN junction semiconductor lasers based on quantum wells usually does not exceed 4 μm, which cannot meet the wavelength requirements of mid-far-infrared. Due to the existence of manufacturing defects, coupled with excessive Auger recombination loss and free carrier absorption loss, the high-quality operation of the laser cannot be guaranteed. The mid-infrared laser with a wide tunable wavelength can be produced by using nonlinear optical phenomena, which originates from the nonlinear polarizability of material. The optical parametric oscillator (OPO) is a typical example of using difference frequency generation (DFG), and it can generate a mid-infrared laser with the frequency by combining a near-infrared laser with a visible laser. The major advantage of OPO systems is their good accessibility to various pulse forms. By using an ultra-short pulse laser in femtosecond scale, it is possible to generate an extremely strong peak power of up to several MW in pulse mode. Such features are quite advantageous for nonlinear microscopy applications. However, the drawback of OPO systems is their less robustness due to its complex optical system, and the tuning range is still limited because of the wavelength accessibility of nonlinear crystals. In addition, the OPO needs a pump laser system for the optical pumping to generate the nonlinear optical process. Thus, the final form of the whole system is inevitably large and not suitable as a portable system. Distributed feedback (DFB) lasers can also generate mid-infrared wavelength and are very useful for spectroscopic sources, but their main disadvantages are their limited tuning capability and lower output power. In order to meet the needs of mid-far-infrared wavelengths, a new type of infrared laser is required, and QCLs are a good choice, which utilize electronic transitions between quantum well subbands instead of interband optical transitions.
The emergence of QCL has created a precedent for the development of mid-far-infrared semiconductor lasers using wide-bandgap materials. Due to its narrow linewidth and high-power operation in the mid-infrared band (3–24 μm) at room temperature continuous wave (CW) conditions, it is very suitable for tracing gas sensing in mid-infrared spectroscopy. At present, quantum well lasers in the mid-infrared band lack continuous wave tunability. Gas lasers, such as CO lasers and CO2 lasers, have a large volume and weight. Lead salt semiconductor lasers have high cooling requirements and low output laser power. QCL overcomes these shortcomings; thus, it can be used in directional infrared countermeasure, gas pollution detection, medical diagnosis, etc. ECQCLs can broaden the working wavelength, improve the beam quality and output laser power, and promote the applications of QCLs.
2. ECQCLs Basic Structure and Characteristics
2.1. Basic Structure of ECQCLs
In general, mid-infrared semiconductor lasers without any other lasers are compact and easy to use; however, their performance is limited because the emission wavelength is not a single laser mode, but it often has multiple laser modes in the spectrum. In addition, the linewidth of each mode is not narrow enough. Therefore, a form of Fabry-Perot (FP) laser is not suitable for spectroscopy applications. An ECQCL is a wavelength tunable laser, which includes an optical gain medium, an optical device for coupling the output of the gain dielectric waveguide to the free space mode of the external cavity, and a wavelength selective component, such as an interference filter or diffraction grating. Other additional optics such as polarizers, beam splitters, and prisms can also be integrated. Compared with FP lasers, ECQCL does not only benefit the tunability, but it also greatly improves the linewidth of the laser.
The basic structure of ECQCLs is usually composed of a laser external cavity and optical feedback elements, such as collimating lenses and wavelength selectors. The wavelength selectors can be a diffraction grating, a photoelectric filter, an acousto-optic filter, etc., and the laser is enhanced through the feedback of the selectors. The spectrum can be reflected by rotating the angle of the grating; thereby, the laser emission wavelength can be adjusted. The basic structure of an ECQCL is shown in Figure 1.
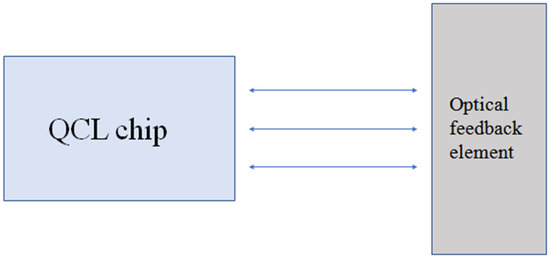
Figure 1. Basic structure of ECQCLs.
2.2. Operating Principle of QCLs
QCL is a unipolar semiconductor device that utilizes electron transitions between the subbands confined in the heterostructure conduction band for light emission. The electron distribution of a QCL needs to be designed to have a population inversion by adjusting the thicknesses of quantum wells and barriers. Injected electrons flow from the upper levels to lower levels by following the sequential wavefunctions, which represents the electron probability. During this process, the electrons optically transit at the “active region”. By piling the active region of the same design in sequence, light emission can be amplified. As a consequence, the light emitted from one active region stimulates the following radiation of photons, achieving laser oscillation. During this process, the electrons flow through from one active region to the next active region while generating the stimulated emission of photons and electrons. This is why the laser device is called a quantum “cascade” laser. The more cascades are built, the more electrons can contribute to the stream for light emission. This optical amplification mechanism gives higher quantum efficiency in the laser operation.
QCLs avoid the operating principle of conventional semiconductor lasers by relying on a radically different process for laser emission, which is independent of the band gap [1]. Instead of using opposite charge carriers in semiconductors (electrons and holes) at the bottom of their respective conduction bands and valence bands, which recombine to produce light of frequency ν ≈ Eg/h (where Eg is the energy band gap and h is Planck’s constant), QCLs use only one type of charge carriers (electrons), which undergo a quantum jump between energy levels En and En−1 to create a laser photon of frequency (En–En−1)/h. The energy diagram of QCLs is shown in Figure 2. These energy levels do not naturally exist in the constituent materials of the active region, but they are artificially created by constructing the active region into nanometer-thick quantum wells. The electron motion perpendicular to the layer interface is quantified and characterized in terms of energy levels, the difference of which is determined by the thickness of the wells and the height of the energy barrier separating the wells. The implications of this new approach are profound. Based on the decoupling of lasing emissions from the bandgap by exploiting optical transitions between quantized electronic states, QCLs are equivalent to a laser with operating characteristics that are quite different from semiconductor lasers and features far superior to semiconductor lasers.
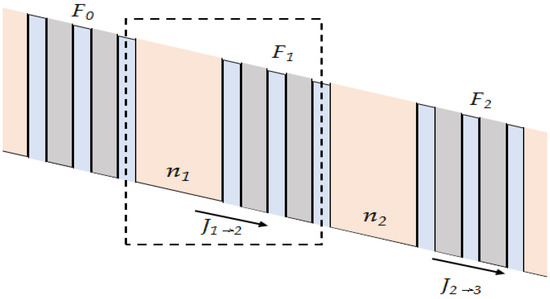
Figure 2. Energy diagram of three well structures of QCL(current densities Jm, electron densities nm and fields Fm, module number m).
3. Applications of Mid-Infrared ECQCLs
ECQCLs have the advantages of high conversion efficiency, compact size, and high reliability [2]. Their lasing wavelength covers two important atmospheric windows of 3–5 μm and 8–14 μm; thus, it can be used in molecular detection, free space communication, and industry applications. Using tunable ECQCL spectroscopy has many advantages, including high sensitivity and selectivity. It is non-destructive, fast, and requires no sample preparation. With advances in ECQCLs in terms of tunability, output power, reliability, and operating temperature, there has been a growing interest in the use of ECQCLs for gas detection by spectroscopic groups. Molecular detection is currently the most studied and widely used field, such as environmental monitoring, emission measurement, remote sensing, medical and life science applications [3], industrial process control, safety, and basic science, all of which benefit from the technological advancements in ECQCLs. Different techniques for absorption spectroscopy were demonstrated using ECQCLs [4][5]. Various substances have unique functional groups, and specific functional groups have specific optical activities and have different absorption characteristics for different wavelength lasers. Therefore, by analyzing the spectral changes caused by the gas, the corresponding molecular content and species can be obtained. Due to the high resolution and high reliability of ECQCLs, there are important application prospects in this field. Laser-based infrared spectroscopy is an emerging key technology for analyzing solutes and monitoring reactions in liquids in real time. Compared to the traditional standard Fourier transform infrared spectroscopy (FTIR), the larger applicable path length enables the robust measurement of analytes in strongly absorbing matrices such as water. Recent advances in laser development have also provided a wide range of accessible spectral coverage, thus overcoming the inherent shortcomings of laser-based infrared spectroscopy.
3.1. Application of ECQCLs in Gas Detection
The detection of gas is important in many applications, including combustion diagnostics, industrial detection, atmospheric detection, and medical detection.
3.1.1. Nitric Oxide (NO) Detection
In 2005, G. Wysocki et al. [6] reported a mode-hopping, broadly tunable CW and thermoelectrically cooled ECQCL capable of high-resolution spectroscopic measurements. The system provides independent wavelength tracking through all three wavelength-selective elements of ECQCLs, which made it suitable for applications using gain chips. The current prototype instrument had a wavelength of 5.2 μm, a tuning range of 35 cm−1, and a continuous mode-hop-free (MHF) mode tuning range of 2 cm−1. The overall performance of the spectrometer system was demonstrated by direct absorption spectroscopy measurements of NO under reduced pressure. Its wavelength modulation capability, coupled with wide tunability and high spectral resolution, made the ECQCL an excellent light source for many mid-infrared spectroscopy applications, such as trace gas detection.
In 2010, V. Spagnolo et al. [7] reported a gas sensor based on quartz-enhanced photoacoustic detection and an ECQCL. It was characterized by NO absorption multiplication at 5.26 μm to monitor trace NO and studied the dependence of signal and noise on gas pressure to optimize the performance of the sensor. The tuning range of the ECQCL was 5.13–5.67 μm, and the specified MHF mode tuning range was 5.26–5.53 μm, which corresponded to 5% of its central wavelength; the output power exceeded 100 mW. By contrast, quartz-enhanced photoacoustic spectroscopy (QEPAS) technology was competitive in sensitivity, while offering a more compact sensor design and smaller size.
3.1.2. CO2 Detection
In 2017, Ramin Ghorbani et al. [8] reported a mid-infrared tunable diode laser absorption spectroscopy sensor for the real-time detection of CO2 in exhaled breath. The system used an ECQCL. The output wavelength tunable range was from 4.50 to 4.96 µm, and the peak output power was 160 mW.
3.1.3. Butane (C4H10) Detection
In 2013, D. Mammez et al. [9] reported the commercialization of an ECQCL in a photoacoustic spectrometer with an emission wavelength of 10.5 μm. The spectrometer could measure in a wide spectral range of 60 cm−1, which means that the spectra of complex molecules as well as the entire absorption bands of small molecules could be recorded. The wide tuning range of this photoacoustic spectrometer light source demonstrates the possibility of detecting complex small molecules such as CO2 and C4H10. Figure 3 shows the structure diagram of spectral detection.
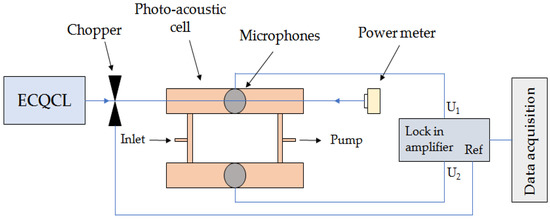
Figure 3. Structure diagram of spectral detection.
3.1.4. Acetylene (C2H2) Detection
In 2018, Abhijit Maity et al. [10] reported a mid-infrared detection strategy using an ECQCL, and the working wavelength was from 7.5 µm to 8 µm. The C2H2 detection had a noise limit of three parts per billion (ppb), and the integration time was 110 s. The current high resolution ECQCL system was further validated in the C2H2 concentration range of 0.1–1000 ppm, which showed good promise in practical sensing applications.
3.1.5. Nitrous Oxide(N2O) Detection
In 2019, Faisal Nadeem et al. [11] reported a mid-infrared trace gas detection system that was used to detect N2O. An ECQCL was used in the system, and the working wavelength was 7.7 µm, the output power was 40 mW, and the tunable range was about 320 cm−1.
3.1.6. Methane (CH4) Detection
In 2017, Abhijit Maity et al. [12] reported mid-infrared CW cavity ring down spectroscopy (CRDS) technology and MHF ECQCL technology operating at 7.5 µm. The authors validated the ECQCL-based high-resolution CW-CRDS system by measuring the 12CH4 and 12CH4 isotopes of CH4 as a reference molecule. By probing the asymmetric bending (υ4 band), vibrations of the 12C and 13C isotopes of CH4 in the sample from bonds centered at 7.534 µm and 7.502 µm, respectively. The current high-resolution CW-CRDS system could further utilize the spectral region covering 7.5–8 µm to trace several other molecular species and their isotopes.
In 2018, Xiaojuan Cui et al. [13] introduced a compact laser absorption sensor system associated with a 152 m long absorption cell for the simultaneous detection of N2O, CH4, and the second harmonic of H2O vapor. An 8 μm ECQCL was the excitation laser source, and three adjacent absorption lines, N2O, CH4, and H2O, at 7.968 µm and 7.969 µm were simultaneously aimed at 7.965 µm. At the optimum pressure of 50 Torr, the lowest detection limit was achieved with 1 s integration time, 0.9 ppb for N2O, 4.8 ppb for CH4, and 31 ppm for H2O.
In 2021, Qianhe Wei et al. [14] reported a gas sensor based on a tunable 7.6 µm CW MHF ECQCL (from 7.4 µm to 7.7 µm) CRDS technique. The sensor could detect CH4 and N2O in ambient air.
3.1.7. Chlorodifluoromethane (CHClF2) Detection
CHClF2 is one of the most abundant HCFCs in the atmosphere. Due to its relatively low ozone depletion potential in chlorine-containing haloalkanes, it is often used as a replacement for the high ozone depleting CFC-11 and CFC-12. CHClF2 is commonly used as a refrigerant in air conditioning systems. Although developed countries are phasing out CHClF2 due to high global warming potential, the use of CHClF2 continues to increase due to a high demand in developing countries. In 2019, Sheng Zhou et al. [15] reported a sensor that used QEPAS and ECQCL to detect CHClF2 with unresolved rotation-vibration absorption lines. The spectral range was from 7.04 µm to 8.13 µm.
3.1.8. Hydrogen Sulfide (H2S) Detection
In 2017, Michal Nikodem et al. [16] reported on a quantum cascade laser-based spectroscopic system for the detection of H2S in the mid-infrared of 7.2 μm, and the wavelength tunable range was from 7 to 8.2 μm.
In 2019, Mithun Pal et al. [17] reported a sensor that used a mid-infrared ECQCL cavity ring-down spectroscopy to simultaneously monitor the 32S, 33S, and 34S isotopes of H2S. It verified the possibility of this system for tracking the characteristics of sulfur isotopes in compounds in practical applications. Nine independent transition lines of H232S and H233S isotopes in the current MHF ECQCL tuning range were further explored for the trace monitoring of the H2S single isotope. At a pressure of 30 Torr and an integration time of 255 s, the lowest detection limit was 20 ppb. It provided a new method that combined the unique spectral features of 7.5 μm, the high sensitivity of CRDS technology, the high resolution of ECQCL, and a wide MHF tunability.
3.2. Protein Detection
Due to the limited emission wavelength range of ECQCLs, the spectral coverage is limited compared to FTIR, but its significant advantage is that it is stable and convenient in the detection of amides [18]. The large optical path can be used to directly measure the infrared absorption spectrum of water-based solutions, such as body fluids (blood, serum, breast milk), foods (commercial milk), etc. Compared to earlier laser-based infrared lasers, the expanded spectral coverage, including the most prominent protein infrared band, provides advantages for qualitative and quantitative studies of proteins. In the future, ECQCLs will be used to study dynamic secondary structure changes and stoichiometry-based protein quantification in complex matrices.
In 2019, Milagros Montermurro et al. [19] reported a rapid analysis system of commercial milk proteins using ECQCL mid-infrared spectroscopy. In the system, a thermoelectrically cooled ECQCL with a repetition rate of 100 kHz and a pulse width of 5000 ns was used. All spectra were recorded in the spectral tuning range of 5.78–6.8 µm, covering the amide I and amide II regions of the protein, with a scan speed of 1200 cm−1s−1. The mid infrared ECQCL was focused on the detector element by a gold-coated off-axis parabolic mirror with a focal length of 43 mm. The operating temperature was −78 °C.
In 2020, Alicja Dabrowska et al. [20] reported a Mach-Zehnder interferometer-based sensor for detecting the dispersive spectroscopy of proteins. This is also the first time that the refractive index spectrum of a protein was measured with such high speed and resolution over such a broad spectral range. The thermoelectrically cooled ECQCL could be tuned in the range of 5.78–6.8 µm. Dispersive spectroscopy achieves a figure of merit similar to established high-end FTIR spectroscopy at the same acquisition time. In the same year, a mid-infrared transmission setup for the analysis of protein amide I and amide II bands in aqueous solution was studied using the ECQCL.
In 2021, Schwaighofer Andreas et al. [21] reported a commercial room temperature operating broadband ECQCL infrared spectroscopy with a spectral coverage of 5.65–7.4 µm combined with FTIR spectroscopy that was compared and demonstrated for its application in measuring a protein secondary structure in water and for monitoring the lipase-catalyzed saponification of triacetin. For the obtained limits of detection, ECQCL-based spectrometers performed better than research-grade FTIR spectrometers with liquid nitrogen cooled detectors. The device monitored the enzymatic hydrolysis of triacetin by lipase, demonstrating the advantage of broad spectral coverage for the subsequent monitoring of complex chemical reactions that cannot be readily obtained by FTIR spectroscopy without the use of liquid nitrogen cooling.
3.3. Industry Detection
In 2020, Mark C. Phillips et al. [22] reported a swept-wavelength ECQCL that was used to perform the standoff detection of combustion gases in a plume generated from an outdoor high-explosive open detonation. The swept-ECQCL system was located at a standoff distance of 830 m from a 41 kg charge of LX-14 (polymer-bonded high explosive) and was used to measure the infrared transmission or absorption through the post-detonation plume as it propagated through the beam path. The swept-ECQCL was operated continuously to record broadband absorption spectra at a 200 Hz rate over a spectral range from 2050 cm−1 to 2230 cm−1. The fitting of measured spectra was used to determine time-resolved column densities of CO, CO2, H2O, and N2O.
In 2020, Anaïs Parrot et al. [23] reported an ECQCL mid-infrared reflectance spectroscopy that was used to discriminate silicate and carbonate minerals in a standoff measurement setting. The tunable ECQCL source that was used allowed measurement from the 5.2 µm to 13.4 µm wavelength, where the fundamental vibrational bands of silicates and carbonates were observed. Mid-infrared reflectance spectroscopy using compact ECQCL sources allowed rapid spectral measurements at standoff distances and high spatial resolution. It showed the potential of ECQCL mid-infrared reflectance spectroscopy for in the field mining applications.
In 2022, Francis Vanier et al. [24] designed an ECQCL-based mid-infrared spectrometer. The light source consisted of four ECQCLs with spectral coverage ranging from 5.2 µm to 13.4 µm wavelengths. The performance of a mid-infrared reflectance spectroscopy device based on a tunable ECQCL module was described. The results assessed the quality and usability of spectra of mineral mixtures obtained using ECQCL-based mid-infrared spectroscopy, completing the first step in mineral characterization using ECQCL-based mid-infrared spectroscopy.
This entry is adapted from the peer-reviewed paper 10.3390/cryst12111564
References
- Capasso, F. High-performance mid-infrared quantum cascade lasers. Opt. Eng. 2010, 49, 1102.
- Niu, S.; Liu, J.; Zhang, J.; Zhuo, N.; Zhai, S.; Wang, X.; Wei, Z. Single-Mode Fabry-Pérot Quantum Cascade Lasers at λ~10.5 µm. J. Mater. Sci. Chem. Eng. 2020, 8, 85–91.
- Risby, T.; Solga, S. Current status of clinical breath analysis. Appl. Phys. A 2006, 85, 421–426.
- Wysocki, G.; Lewicki, R.; Curl, R.; Tittel, F.; Diehl, L.; Capasso, F.; Troccoli, M.; Höfler, G.; Bour, D.; Corzine, S.; et al. Widely tunable mode-hop free external cavity quantum cascade lasers for high resolution spectroscopy and chemical sensing. Appl. Phys. B 2008, 92, 305–311.
- Kosterev, A.; Wysocki, G.; Bakhirkin, Y.; So, S.; Lewicki, R.; Fraser, M.; Tittel, F.; Curl, R. Application of quantum cascade lasers to trace gas analysis. Appl. Phys. 2008, 690, 165–176.
- Wysocki, G.; Curl, R.; Tittel, F.; Maulini, R.; Bulliard, J.; Faist, J. Widely tunable mode-hop free external cavity quantum cascade laser for high resolution spectroscopic applications. Appl. Phys. B 2005, 81, 769–777.
- Spagnolo, V.; Kosterev, A.A.; Dong, L.; Lewicki, R.; Tittel, F.K. NO trace gas sensor based on quartz-enhanced photoacoustic spectroscopy and external cavity quantum cascade laser. Appl. Phys. B 2010, 100, 125–130.
- Ramin, G.; Schmidt, F.M. Real-time breath gas analysis of CO and CO2 using an EC-QCL. Appl. Phys. B 2017, 123, 144.
- Mammez, D.; Stoeffler, C.; Cousin, J.; Vallon, R.; Mammez, M.; Joly, L.; Parvitte, B.; Zéninari, V. Photoacoustic gas sensing with a commercial external cavity-quantum cascade laser at 10.5 µm. Infrared Phys. Technol. 2013, 61, 14–19.
- Maity, A.; Pal, M.; Maithani, S.; Banik, G.D.; Pradhan, M. Wavelength modulation spectroscopy coupled with an external-cavity quantum cascade laser operating between 7.5 and 8 µm. Laser Phys. Lett. 2018, 15, 045701.
- Nadeem, F.; Khodabakhsh, A.; Mandon, J.; Cristescu, S.S.; Harren, F.J.M. Detection of N2O Using An External-Cavity Quantum Cascade Laser. OSA Contin. 2019, 2, 2667–2682.
- Maity, A.; Pal, M.; Banik, G.D.; Maithani, S.; Pradhan, M. Cavity ring-down spectroscopy using an EC-QCL operating at 7.5 m for direct monitoring of methane isotopes in air. Laser Phys. Lett. 2017, 14, 115701.
- Cui, X.; Dong, F.; Zhang, Z.; Sun, P.; Xia, H.; Fertein, E.; Chen, W. Simultaneous detection of ambient methane, nitrous oxide, and water vapor using an external-cavity quantum cascade laser. Atmos. Environ. 2018, 189, 125–132.
- Wei, Q.; Li, B.; Wang, J.; Zhao, B.; Yang, P. Impact of Residual Water Vapor on the Simultaneous Measurements of Trace CH4 and N2O in Air with Cavity Ring-Down Spectroscopy. Atmosphere 2021, 12, 221.
- Zhou, S.; Xu, L.; Zhang, L.; He, T.; Liu, N.; Liu, Y.; Yu, B.; Li, J. External cavity quantum cascade laser-based QEPAS for chlorodifluoromethane spectroscopy and sensing. Appl. Phys. B 2019, 125, 125.
- Nikodem, M.; Krzempek, K.; Stachowiak, D.; Wysocki, G. Quantum cascade laser-based analyzer for hydrogen sulfide detection at sub-parts-per-million levels. Opt. Eng. 2017, 57, 011019.
- Pal, M.; Maithani, S.; Maity, A.; Pradhan, M. Simultaneous monitoring of 32S, 33S and 34S isotopes of H2S using cavity ring-down spectroscopy with a mid-infrared external-cavity quantum cascade laser. J. Anal. At. Spectrom. 2019, 34, 860–866.
- Schwaighofer, A.; Montemurro, M.; Freitag, S.; Kristament, C.; Culzoni, M.J.; Lendl, B. Beyond FT-IR Spectroscopy EC-QCL based mid-IR Transmission Spectroscopy of Proteins in the Amide I and Amide II Region. Anal. Chem. 2018, 90, 7072–7079.
- Montemurro, M.; Schwaighofer, A.; Schmidt, A.; Culzoni, M.J.; Mayer, H.K.; Lendl, B. High-throughput quantitation of bovine milk proteins and discrimination of commercial milk types by external cavity-quantum cascade laser spectroscopy and chemometrics. Analyst 2019, 144, 5571–5579.
- Dabrowska, A.; Schwaighofer, A.; Lindner, S.; Lendl, B. Mid-IR refractive index sensor for detecting proteins employing an external cavity quantum cascade laser-based Mach-Zehnder interferometer. Opt. Express 2020, 28, 36632–36642.
- Schwaighofer, A.; Akhgar, C.K.; Lendl, B. Broadband laser-based mid-IR spectroscopy for analysis of proteins and monitoring of enzyme activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 253, 119563.
- Phillips, M.C.; Harilal, S.S.; Yeak, J.; Jason Jones, R.; Wharton, S.; Bernacki, B.E. Standoff detection of chemical plumes from high explosive open detonations using a swept-wavelength external cavity quantum cascade laser. J. Appl. Phys. 2020, 128, 163103.
- Parrot, A.; Vanier, F.; Blouin, A. Standoff mid-infrared reflectance spectroscopy using quantum cascade laser for mineral identification. SPIE Future Sens. Technol. 2020, 11525, 217–225.
- Vanier, F.; Parrot, A.; Padioleau, C.; Blouin, A. Mid-Infrared Reflectance Spectroscopy Based on External Cavity Quantum Cascade Lasers for Mineral Characterization. Appl. Spectrosc. 2022, 76, 361–368.
This entry is offline, you can click here to edit this entry!
