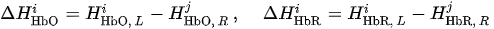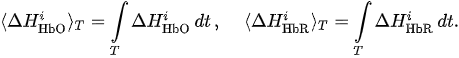Sensor-level human brain activity is studied during real and imaginary motor execution using functional near-infrared spectroscopy (fNIRS). Blood oxygenation and deoxygenation spatial dynamics exhibit pronounced hemispheric lateralization when performing motor tasks with the left and right hands. This fact allowed us to reveal biomarkers of hemodynamical response of the motor cortex on the motor execution, and use them for designing a sensing method for classification of the type of movement. The recognition accuracy of real movements is close to 100%, while the classification accuracy of imaginary movements is lower but quite high (at the level of 90%). The advantage of the proposed method is its ability to classify real and imaginary movements with sufficiently high efficiency without the need for recalculating parameters. The proposed system can serve as a sensor of motor activity to be used for neurorehabilitation after severe brain injuries, including traumas and strokes.
- brain activity
- functional near-infrared spectroscopy (fNIRS)
- real and imaginary motor execution
- sensor level
1. Introduction
From the viewpoint of nonlinear dynamics, brain is a very complex dynamical system, containing around 86 billion neurons [1]. The neurons are connected by synapses, thus forming a complex network with nodes and links represented respectively by neurons and synapses. Specific features of time-spatial activity of the neural network in the brain cortex and cooperative dynamics of different brain areas (e.g., event-related synchronization/desynchronization) on a sensor level provide important information about the current state of the nervous system and cognitive brain ability [2]. Particular brain states are associated with motor brain activity during either real or imaginary movement.
Revealing specific features of spatial brain cortex activity related to real motions and motor imagery of different limbs can be essential not only for basic research in neuroscience, but also for applications in medicine to improve the quality of life of post-traumatic and post-stroke patients using brain-computer interfaces (BCI) for rehabilitation [3][4][5] or to control prostheses and exoskeletons [6]. One of the important BCI functions is online detection of specific features of electromagnetic brain activity using electroencephalography (EEG) or magnetoencephalography (MEG), and transformation of certain patterns into control commands to perform specific actions in the environment without the need of “classical” methods of human–machine interaction.
Apart from EEG and MEG, other methods are also used to acquire information about brain states. In particular, functional near-infrared spectroscopy (fNIRS) [7][8] is a powerful tool of noninvasive optical imaging successfully used in BCI for registration of brain activity and control command formation [9][10]. Control commands for this kind of BCI should not be affected by any muscular activity [11]. Therefore, a study of brain states related to motor imagery is very important for designing such BCI [12][13]. Motor imagery is a mental process by which a person rehearses or simulates a given action with no real motor activity. Some researchers treat motor imagery as a conscious application of unconscious preparation for real motor activity [14]. A number of studies have highlighted common features for real and imaginary motor activity [15][16][17]. One of the common features, important for the BCI development, is that the cortical layout in the primary motor cortex M1 is quite similar between motor execution and motor imagery.
2. Participants
Twelve healthy volunteers (age: 22–38 years, gender: 7 men and 5 women), right-handed, amateur practitioners of physical exercises, non-smokers participated in the experiment. None of the subjects had diagnosed diseases of the musculoskeletal system and neurological diseases and did not take medications. Every participant was asked to maintain a healthy lifestyle with 8-hours night rest for 48 h before starting the experiment.
Each participant provided informed written consent before participating in the experiment. The experimental procedure was performed in accordance with the Helsinki’s Declaration and approved by the local Ethics Committee of the Innopolis University.
3. Experimental Equipment
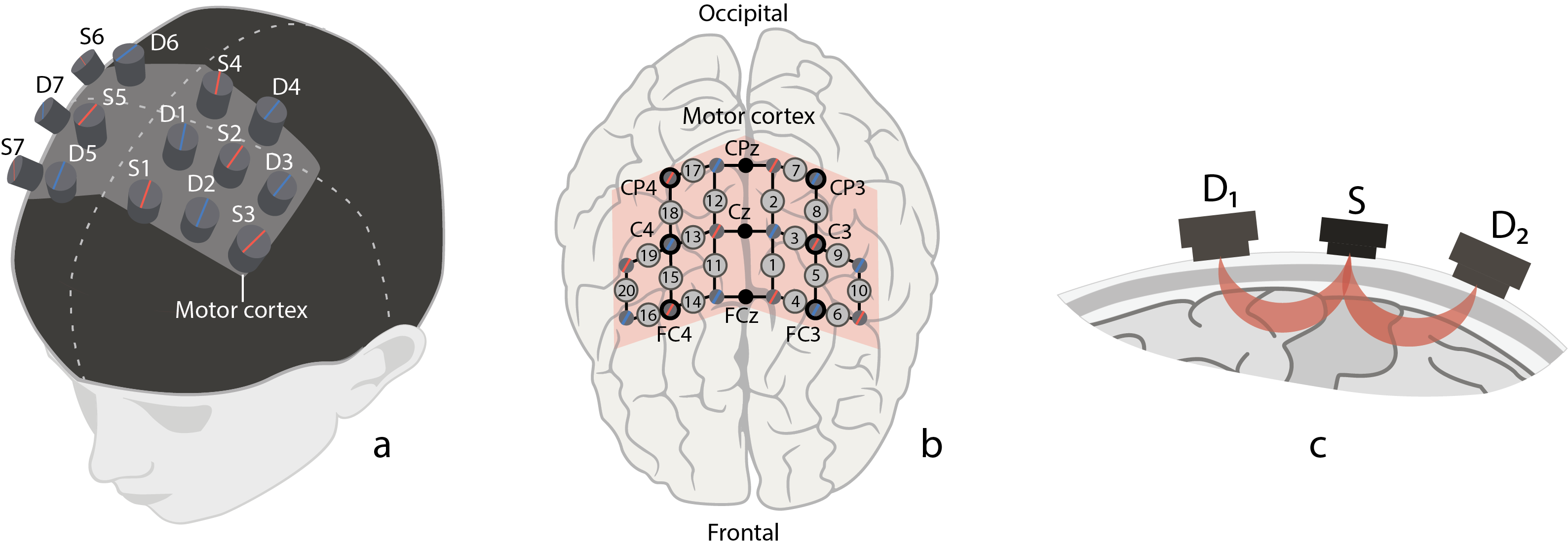
The experiment was designed to record a hemodynamic neuronal response in the motor cortex using fNIRS which records fast changes in the brain activity. The fNIRS signals were acquired by the NIRScout device manufactured by the NIRx Company (Germany). The NIRScout system has a 7.8125-Hz resolution and contains 8 sources and 8 detectors placed on the subject’s scalp in the primary motor cortex area (M1) as shown in Figure 1a. Each pair “source–detector” was placed close enough to each other (about 3 cm) to form a fNIRS channel. In our experiments, we used 20 fNIRS channels as illustrated in Figure 1b.
Figure 1. (a) Location of fNIRS sources (marked with red) and detectors (marked with blue) on the subject’s head in the area of primary motor cortex (shaded area). (b) Location of 20 fNIRS channels (grey circles with channel numbers) across motor cortex (shaded area) and 9 EEG electrodes (CP4, CPz, CP3, C4, Cz, C3, FC4, FCz, FC3) according to “10–10” scheme (black circles with channel names). (c) Schematic illustration of traveling path of near-infrared light from source (S) to neighbour detectors (D1 and D2) through brain cortex matter.
All experiments were carried out in the Neuroscience and Cognitive Technology Lab of the Innopolis University.
4. Experimental Procedure
The experiment was performed as follows. The subjects were sitting on a comfortable chair while performing motor actions or motor imaginary of left and right hands according to the corresponding text command on a computer monitor placed in front of the subject’s eyes at a distance of 70–80 cm.
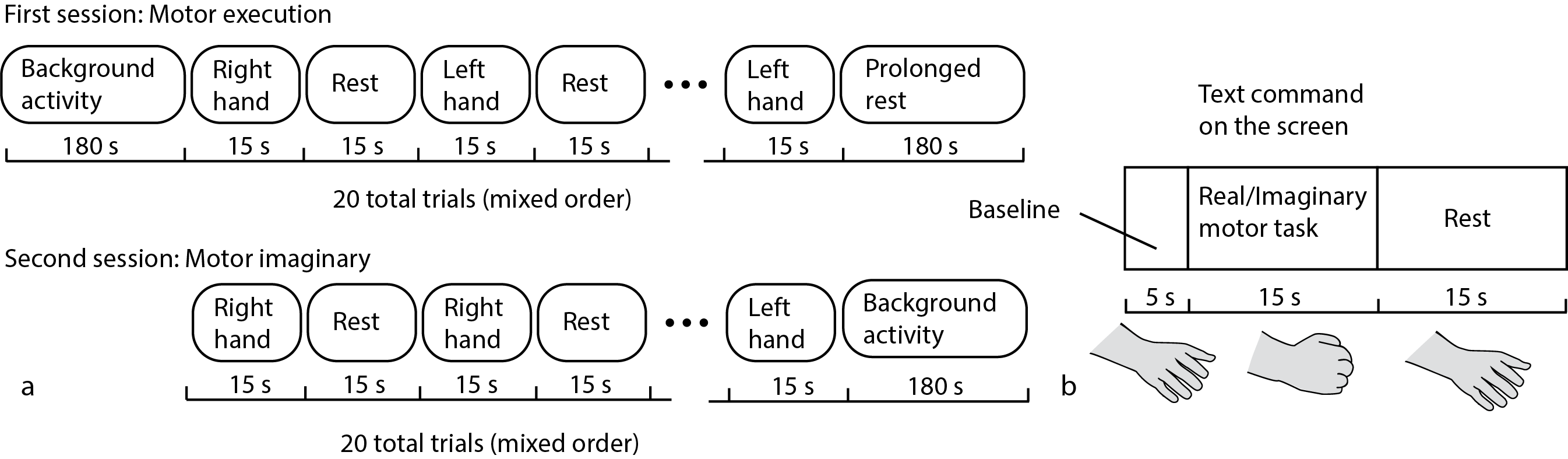
Figure 2. Schematic representation of (a) experimental design and (b) task execution during a long fNIRS trial.
The experimental design is schematically shown in Figure 2. Each experiment began and ended with a 3-min recording of background brain activity, during which the subject were instructed to relax and make no hand movements. The experiment included two sessions (Figure 2a). In the first session, the subject was asked to perform real movements with left or right hand according to the corresponding command on the screen. Then, after a short break, in the second session the subject was asked to imagine the same type of movement according to the corresponding command on the screen. Each fNIRS trial during every session consisted of the text command presentation indicating the type of motor activity (the subject was given 15 s to perform required movement) and the rest interval (15 s from the end of motor activity till the next command). There were 10 trials for each type of motor activity. Hand movement consisted of repeated bending/unbending of fingers to the center of the palm (similar to clenching of imaginary ball), as illustrated in (Figure 2b). The repeated movements were performed at the pace comfortable for the subject.
5. Data Acquisition and Pre-Processing
The fNIRS data acquisition and pre-processing procedure were performed with software NIRScout. It is well-known that experimental fNIRS data are often affected by side physiological noises and artifacts, whose characteristic frequencies are in the fNIRS frequency band, including Mayer wave (with a typical frequency close to 0.1 Hz), respiration (close to 0.25 Hz), and heartbeat (close to 1 Hz). As was mentioned in the review paper [18], in many cases the band-pass filtering is mostly sufficient for removing low-frequency physiological noise in fNIRS data. According to this observation, we also applied the 0.01–0.1 Hz band-pass filter to the fNIRS signals using NIRScout to prevent the effect of side physiological activities.
6. Algorithm for Classification of Brain Activity During Real and Imagery Motor Executions
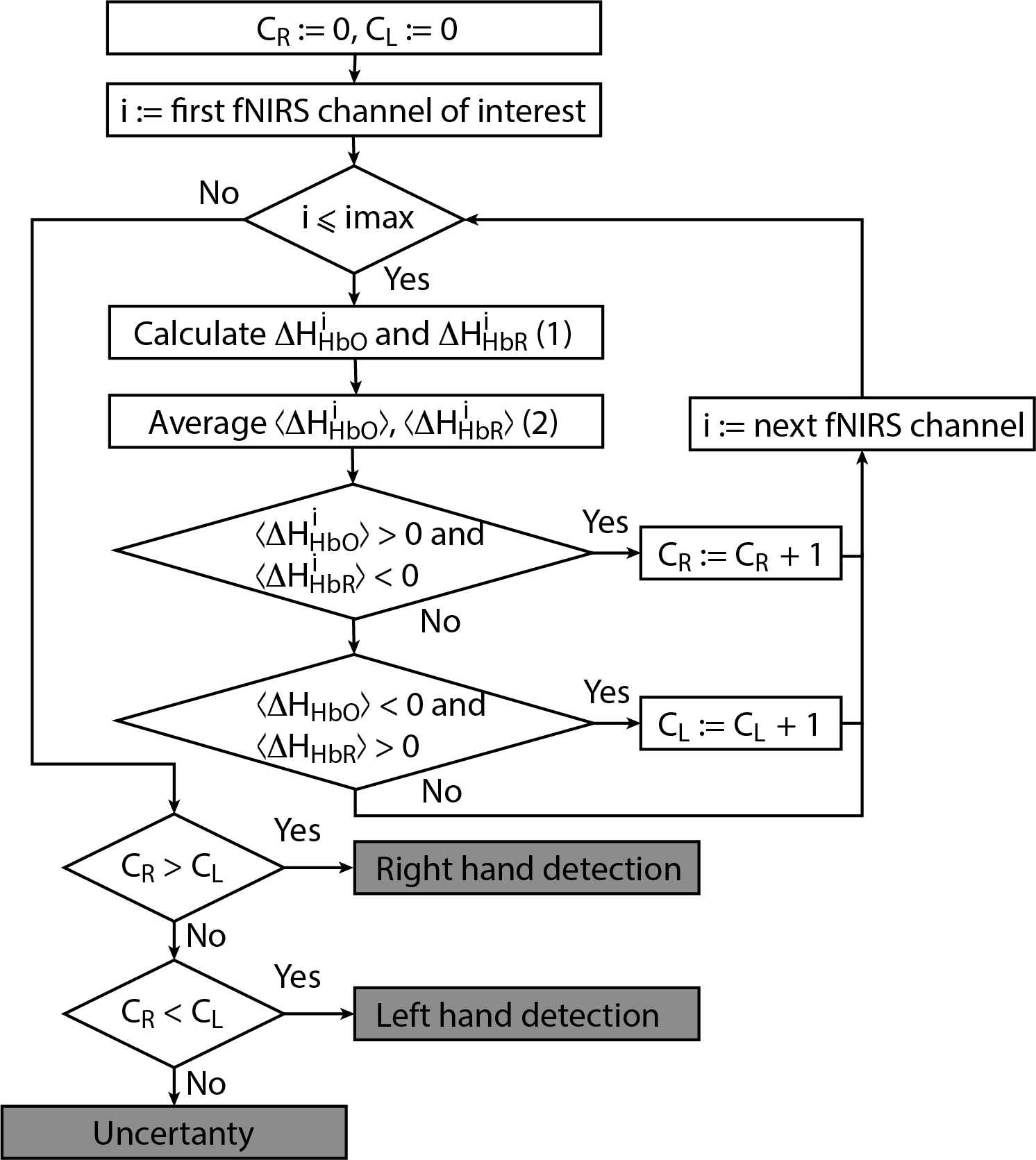
Let us now consider the online algorithm based on the construction of a decision tree for binary classification of brain activity during real and imagery motor executions. The tree-like model of decisions was created as a result of empirical analysis of the experimental data. The proposed algorithm for processing fNIRS data is illustrated in Figure 3 in the form of a flowchat which contains the following main steps.
Figure 3. Flowchart of online classification algorithm for fNIRS signals corresponding to left/right-hand movement. The main advantage of the algorithm is that it can be used for both real and imaginary movements.
For each considered channel i and type of motor activity (right/left hand, execution/imagery) we subtract spatial oxyhemoglobin (HbO) (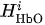
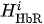
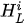
Then, we average 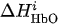
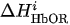
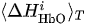
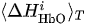
For each separate fNIRS signal trial, we calculate characteristics CR and CL taking into account the following criteria for each considered symmetric fNIRS channels in the left and right hemispheres.
(i) If 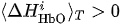
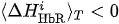
(ii) If 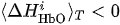
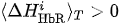
Finally, we make a decision according to the following criteria.
(i) If CR > CL, then right-hand (real or imaginary) motor activity takes place.
(ii) If CR < CL, then left-hand (real or imaginary) activity takes place.
(iii) If CR = CL, then the type of activity is uncertain.
7. Results
We implemented the classification algorithm described above in our fNIRS-based experimental system for online classification of real and imaginary motor actions. We used the proposed classifier with six fNIRS channels i = {2, 7, 8} in the left hemisphere and j = {12, 17, 18} symmetric channels in the right hemisphere. Notably, in the majority of cases the type of motor action (both real and imagery) in all subjects was correctly identified by the data from only three fNIRS channels. Table 1 shows the results of automatic classification of left/right hand real and imaginary movements, as well as statistical analysis of true positive fraction, true negative fraction, and false positive fraction.
Table 1. The results of automatic classification of different types of motor action (mean ± S.D.) using i = {2, 7, 8} and j = {12, 17, 18} fNIRS channels in the left and right hemisphere, respectively.
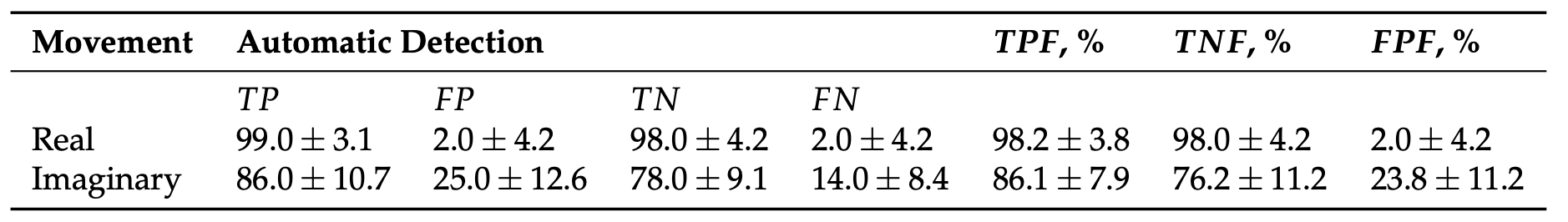
The proposed fNIRS-based method provides close to 100% recognition accuracy in the detection of real movements, while the classification accuracy of motor imagery is a little smaller and reach 90%.
The important advantage of the proposed method is the possibility to efficiently classify different types of movement, both real and imaginary, without recalculation of the system parameters. This essential feature of the developed sensor results from pronounced laterality of the hemodynamic brain response to motor activity.
The knowledge of the hemodynamic behavior in the motor cortex during real and imaginary motor activity along with approaches for its detection can be helpful not only for fundamental studies on human motor-related tasks but also for the development of fNIRS-based BCIs.
This entry is adapted from the peer-reviewed paper 10.3390/s20082362
References
- Suzana Herculano-Houzel; The Human Brain in Numbers: A Linearly Scaled-up Primate Brain. Frontiers in Human Neuroscience 2009, 3, 31, 10.3389/neuro.09.031.2009.
- Niedermeyer, E.; Fernando, L.S. . Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia , 2004; pp. 1309.
- Ethan Buch; Cornelia Weber; Leonardo G. Cohen; Christoph Braun; Michael Dimyan; Tyler Ard; Jurgen Mellinger; Andrea Caria; Surjo R. Soekadar; Alissa Fourkas; et al. Think to move: a neuromagnetic brain-computer interface (BCI) system for chronic stroke.. Stroke 2008, 39, 910-7, 10.1161/STROKEAHA.107.505313.
- Wei-Peng Teo; Effie Chew; Is Motor-Imagery Brain-Computer Interface Feasible in Stroke Rehabilitation?. PM&R 2014, 6, 723-728, 10.1016/j.pmrj.2014.01.006.
- Ujwal Chaudhary; Niels Birbaumer; Ander Ramos-Murguialday; Brain–computer interfaces for communication and rehabilitation. Nature Reviews Neurology 2016, 12, 513-525, 10.1038/nrneurol.2016.113.
- Michael R Tucker; Jeremy Olivier; Anna Pagel; H. Bleuler; Mohamed Bouri; Olivier Lambercy; Jose Del R. Millan; Robert Riener; Heike Vallery; Roger Gassert; et al. Control strategies for active lower extremity prosthetics and orthotics: a review. Journal of NeuroEngineering and Rehabilitation 2015, 12, 1, 10.1186/1743-0003-12-1.
- Androu Abdalmalak; Daniel Milej; Loretta Norton; Derek B. Debicki; Teneille Gofton; Mamadou Diop; Adrian M. Owen; Keith St. Lawrence; Single-session communication with a locked-in patient by functional near-infrared spectroscopy. Neurophotonics 2017, 4, 1, 10.1117/1.NPh.4.4.040501.
- Androu Abdalmalak; Daniel Milej; Mamadou Diop; Mahsa Shokouhi; Lorina Naci; Adrian M. Owen; Keith St. Lawrence; Can time-resolved NIRS provide the sensitivity to detect brain activity during motor imagery consistently?. Biomedical Optics Express 2017, 8, 2162-2172, 10.1364/BOE.8.002162.
- Yohei Tomita; François-Benoit Vialatte; Gérard Dreyfus; Yasue Mitsukura; Hovagim Bakardjian; Andrzej Cichocki; Bimodal BCI Using Simultaneously NIRS and EEG. IEEE Transactions on Biomedical Engineering 2014, 61, 1274-1284, 10.1109/tbme.2014.2300492.
- Power S. D.; Kushki A.; Chau T.; Automatic single-trial discrimination of mental arithmetic, mental singing and the no-control state from prefrontal activity: toward a three-state NIRS-BCI. BMC research notes, 2012, 5, 141, 10.1186/1756-0500-5-141.
- Luis Fernando Nicolas-Alonso; Jaime Gomez-Gil; Brain Computer Interfaces, a Review. Sensors 2012, 12, 1211-1279, 10.3390/s120201211.
- Parth Chholak; Guiomar Niso; Vladimir A. Maksimenko; Semen A. Kurkin; Nikita S. Frolov; Elena Pitsik; Alexander E. Hramov; Alexander N. Pisarchik; Visual and kinesthetic modes affect motor imagery classification in untrained subjects.. Scientific Reports 2019, 9, 9838, 10.1038/s41598-019-46310-9.
- S. Lemm; C. Schäfer; G. Curio; BCI Competition 2003—Data Set III: Probabilistic Modeling of Sensorimotor$mu$Rhythms for Classification of Imaginary Hand Movements. IEEE Transactions on Biomedical Engineering 2004, 51, 1077-1080, 10.1109/tbme.2004.827076.
- M. Jeannerod; Mental imagery in the motor context. Neuropsychologia 1995, 33, 1419-1432, 10.1016/0028-3932(95)00073-c.
- Jorn Munzert; Britta Lorey; Karen Zentgraf; Cognitive motor processes: The role of motor imagery in the study of motor representations. Brain Research Reviews 2009, 60, 306-326, 10.1016/j.brainresrev.2008.12.024.
- Nikhil Sharma; P.S. Jones; T.A. Carpenter; Jean Claude Baron; Mapping the involvement of BA 4a and 4p during Motor Imagery. NeuroImage 2008, 41, 92-99, 10.1016/j.neuroimage.2008.02.009.
- Ana Solodkin; Petr Hluštík; E. Elinor Chen; Steven L. Small; Fine Modulation in Network Activation during Motor Execution and Motor Imagery. Cerebral Cortex 2004, 14, 1246-1255, 10.1093/cercor/bhh086.
- Noman Naseer; Keum-Shik Hong; Corrigendum “fNIRS-based brain-computer interfaces: a review”. Frontiers in Human Neuroscience 2015, 9, 172, 10.3389/fnhum.2015.00172.