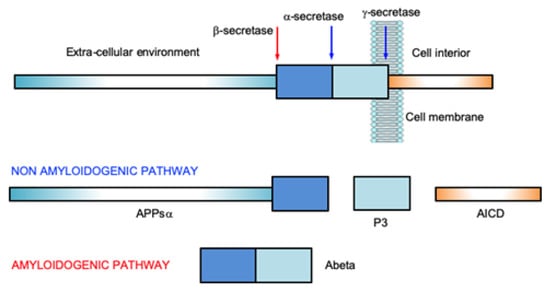
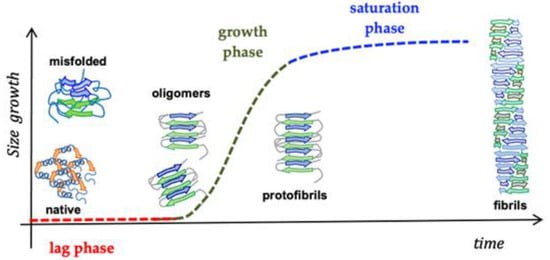
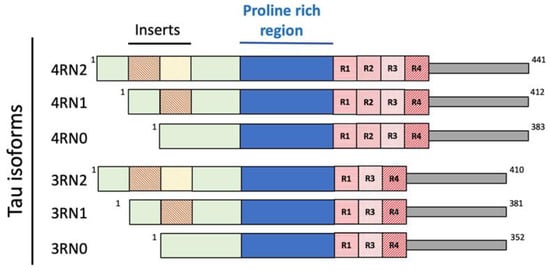
Nanostructured peptides with metal binding properties are promising therapeutic advancements in neurodegenerative diseases. These nanostructures interact with metal ions and influence the biological properties of several proteins involved in neurodegenerative diseases [
]. The brain copper imbalance plays an important role in Abeta aggregation and in AD neurotoxicity. Moreover, the Cu
ion bound to Abeta can induce ROS production. Histidine-containing peptides and proteins are excellent metal binders and are found in many natural systems. For this aim, Caballero and Collaborators studied three short peptides, HWH, HKCH and HAH, forming highly stable albumin-like complexes, with higher affinity for Cu
than for Abeta(1–40). These copper-chelating peptides were designed with the aim of reducing copper toxicity in AD. Furthermore, HWH, HKCH and HAH act as very efficient inhibitors of copper-mediated generation of ROS and prevent the copper-induced overproduction of toxic oligomers in the early stages of amyloid aggregation in the presence of Cu
].
Increasing evidence indicates that oxidative activity may be involved in the etiology of AD as well as other neurodegenerative pathologies and cancer. Under physiological conditions, free radicals, reactive oxygen species (ROS) and reactive nitrogen species (NOS), are normally produced in living cells; just consider, for example, the molecular species generated during the mitochondrial electron transport chain (ETC) and the Krebs cycle [
75]. These unstable molecules, with unpaired electrons, initiate a series of reactions leading to the oxidation of proteins, lipids, and nucleic acids. However, in several cases and at low-to-moderate concentrations, free radicals play a physiological role [
76]. ROS derived by the action of NADPH oxidase, a superoxide-oxidase enzyme, can fight the bacterial infection in the neutrophil phagosome [
77]. Furthermore, ROS are physiologically involved in some cellular pathway signaling and in the regulation of the vascular tone, cell adhesion and apoptosis [
76]. They also have a key role in the protection of adults and embryonic stem cells [
78].
In healthy individuals, the excess production of free radical concentration is counteracted by the oxidative defense system, including glutathione, arginine, and citrulline; some chemical elements such as selenium and zinc; the vitamins A, C and E; the enzymes superoxide dismutase, catalase, glutathione reductase and glutathione peroxidases [
79]. Aging and age-related diseases contribute to the free radical productions [
80].
4. The Antioxidant Properties of Egg-Derived Peptides
Proteins are huge biomolecular and macromolecular structures made up of one or more long chains of amino acid residues. Proteins serve a wide range of roles within animals, including catalyzing metabolic reactions, providing structure to cells and organisms, DNA replication, transporting chemicals, and responding to stimuli. A polypeptide is a linear chain of amino acid residues. Short polypeptides with fewer than 20–30 residues are rarely regarded as proteins and are often referred to as peptides; this is why peptides can be created by the enzymatic digestion of proteins.
Egg white-derived peptides, DHTKE (Asp-His-Thr-Lys-Glu), FFGFN (Phe-Phe-Glu-Phe-His), and MPDAHL (Met-Pro-Asp-Ala-His-Leu), formed via alcalase, were discovered to have antioxidant properties [92,94]. The egg white hydrolyzed by “protease P” give rise to two strongly antioxidant peptides, AEERYP (Ala-Glu-Glu-Arg-Tyr-Pro) and DEDTQAMP (Asp-Glu-Asp-Thr-Gln-Ala-Met-Pro). Pepsin hydrolyzed ovalbumin-derived peptide Tyr-Ala-Glu-Glu-Arg-Tyr-Pro-Ile-Leu has previously been reported to have angiotensin converting enzyme (ACE)-inhibitory activity and showed radical scavenging activity [92,95]. Two antioxidant tetrapeptides (Trp-Asn-Ile-Pro and Gly-Trp-Asn-Ile) were attained from the pyrolytic hydrolyzate of ovotransferrin [96]. Trp-Asn-Ile was proposed as a peptide motif involved in the significant activity of the above tetrapeptides. The ovotransferrin-derived tripeptide Ile-Arg-Trp exhibited powerful radical scavenging activity due to the tryptophan and the peptide bond between Trp and Arg [86,92]. Ovomucin-derived pentapeptide Trp-Asn-Trp-Ala-Asp has been found to decrease H2O2-induced oxidative stress in human fetal kidney cells (HEK-293) by hindering intracellular ROS accumulation. On the other side, from egg yolk, phosvitin phosphopeptides (PPP) obtained from tryptic digestion of phosvitin presented a protective effect against H2O2-induced oxidative stress in human intestinal epithelial cells [92] and, compared with intact phosvitin, PPP has shown a powerful ability to prevent lipid oxidation in the linoleic acid system and more efficient free radical capture [97].
5. Cholinesterase and BACE Inhibitory Activity of Egg-Derived Peptides
The cholinergic loss is one of the most prominent components of the neuropathology of Alzheimer’s disease. The cholinergic system is important for neuronal functions such as memory and learning by playing a main role in promoting neuronal plasticity. The cholinergic hypothesis considers that the level of acetylcholine in the brain of AD patients is low. This can happen because of the degradation produced by two cholinesterases: the first one is the true cholinesterase, AChE, and the other one is a pseudo-cholinesterase, BChE [98]. The hypothesis has received convincing validations, as AChE inhibitors are currently the most prescribed class of drugs for the treatment of AD [99].
Among the four peptides, KLPGF (at the concentration of 50 μg/mL) showed the greatest AChE and BChE inhibitory activity, with inhibition values of 61.23 ± 4.73% and 3.29 ± 0.93%, respectively. Peptide TNGIIR exhibited modest AChE and BChE inhibition with the value of 58.02 ± 1.89% and 1.50 ± 0.24%, respectively. Peptides QIGLF and RVPSL had no noteworthy AChE and BChE inhibitory properties. Furthermore, the peptide KLPGF made a number of powerful hydrogen bonds with numerous important amino acid residues situated in the catalytic and allosteric sites of AChE and a number of hydrophobic interactions with AChE. The contacts between KLPGF and AChE mostly involved the resulting amino acid residues: Tyr70-Trp84-Gly118-Gly119-Trp279-Asp285-Ser286-Ile287-Phe330-Phe331-Tyr334-His440-Gly441 [99].
6. Beta-Sheet Breaker (BSB) Peptides as Abeta Aggregation-Inhibitor
Significant evidence indicated that the key pathological event in Alzheimer’s disease is the switch from a normal soluble Abeta into beta-sheet-rich oligomeric structures which have the capacity to form insoluble amyloid deposits with neurotoxic effects in the brain. Thus, an attractive approach against AD is the inhibition of the aggregation of Abeta through the insertion of different-sized molecules able to prevent fibril formation [
102].
Thus, several studies have been based on the design of a wide range of compounds, from small peptides to large chaperones, to develop inhibitors of Abeta aggregation [
103,
104].
In the late 1990s, Soto and coworkers reported the results of the in vitro addition of different concentrations of a five-residue synthetic peptide, called Beta-Sheet Breaker (BSB), in the solution containing Abeta40 molecules capable of impeding their aggregation [
105].
BSBs represent a class of compounds intended to bind Abeta in specific ways to inhibit and/or block its pathological conformational modification and growth. There are several causes that trigger Abeta formation, and among these are pH changes, apolipoprotein E (ApoE), especially its E4 isoform [
106], α1-antichymotrypsin [
106], and C1q complement factor [
107], oxidative stress [
108], metals [
109], and proteoglycans [
110].
Many distinct small compounds have been shown to avoid and/or annul Abeta polymerization in vitro, unfortunately they lack specificity, a clear mechanism of action, and sometimes show high toxicity, making them difficult to improve and to clinically use [
111].
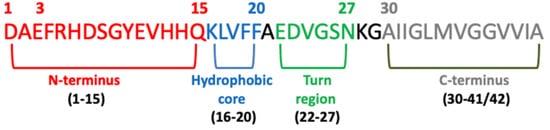
Several studies have confirmed that different Abeta peptide regions contribute in a different way to aggregation and have shed light on several important interactions among specific peptide regions that control this process and are crucial for the peptide’s ability to aggregate and promote neurotoxicity. These regions are: the N-terminus (fragment 1–15) [
112], the hydrophobic core (fragments 16–20) [
113], the hinge or turn regions (fragments 22–27), [
114] and the C-terminus (fragments 31–40/42) [
115] (
Figure 4).
Figure 4. Schematic view of the Abeta domains.
7. The Blood–Brain Barrier (BBB) and AD
The term blood–brain barrier describes the exclusive properties of the central nervous system microvasculature. These central nervous system vessels are non-fenestrated continuous vessels that contain some supplementary properties that allow them to tightly regulate the movement of cells, molecules and ions between the central nervous system and the blood [124]. Thus, BBB endothelial cells tightly regulate central nervous system homeostasis thanks to heavily restricting barrier capacity. This function is critical for proper neuronal function and to protect the central nervous system from injury, toxins, disease, pathogens, and inflammation [125]. The selective and restrictive proprieties of the BBB are an obstacle for drug delivery to the central nervous system. Today, the BBB is thought of as a complex and dynamic interface rather than as a static barrier [126].
Although the mechanism linking Abeta accumulation and BBB dysfunction is poorly explained, it appears clear that the latter causes increased production of Abeta by activation of the β- and γ-secretase activity, establishing a vicious circle [
127]. Furthermore, once that barrier is disrupted, this dysfunction leads to altered signaling homeostasis, ion dysregulation, as well as the entry of immune cells and molecules into the central nervous system. This process leads to neuronal dysfunction and degeneration. Therefore, large compounds easily invade the blood–brain barrier and normally the use of specific drug delivery systems is unnecessary.
8. The Insulin Effect against AD
Insulin, peptide secreted by the pancreas, plays an important role in the regulation of the glucose metabolism in the peripheral tissues. The brain was once considered an insulin-insensitive organ, but today the insulin receptors are present throughout the brain and play a vital role for brain functioning [128]. Human and animal studies indicate that insulin influences cerebral bioenergetics, enhances synaptic viability and dendritic spine formation, increases the turnover of neurotransmitters, and modulates vascular function through effects on vasoreactivity, lipid metabolism, and inflammation [128].
Insulin resistance is a key risk factor for AD [
135,
136,
137]. Studies showed that peripheral insulin resistance in AD patients was positively correlated with Abeta deposition in the brain [
138,
139]. In this context, obese patients with insulin resistance have a higher risk of developing AD [
1]. Reduced levels of IR and a reduced affinity of the receptor for insulin in the brain have been reported in patients with AD compared to controls [
140]. Abeta induced cerebral insulin resistance with effects on insulin signaling by competing, reducing the affinity of insulin binding to its own receptor, or regulating intracellular signalling [
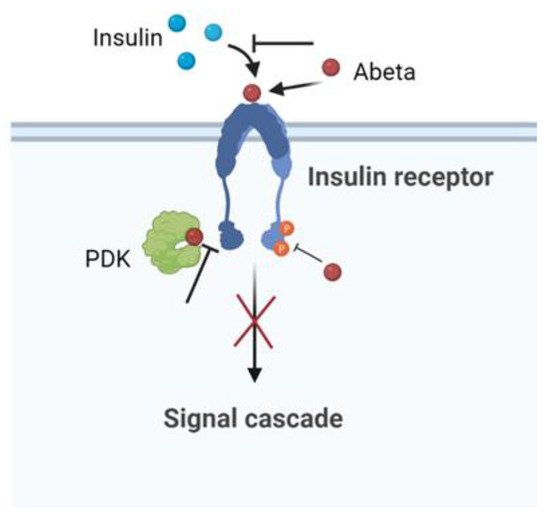
141]. The abeta–IR interaction provoked the inhibition of the
p-Akt insulin survival pathway. Moreover, in vitro experiments indicated that Abeta interrupted insulin signaling by blocking the association between PDK and Akt [
142]. Using cultured hippocampal neurons, the amyloid derived diffusible ligands (ADDLs), that is, the soluble oligomeric forms of Abeta aggregates with the most toxic effect, were found to cause rapid redistribution of IR between the cell body and dendrites. Furthermore, the neuronal response to insulin, measured by the autophosphorylation of IR, was significantly inhibited by the presence of ADDLs. These findings suggested that insulin resistance in the AD brain is a response to Abeta, which disrupted the insulin pathway and caused a brain form of diabetes [
143]. The schematic effect of Abeta in inducing impaired neuronal insulin signaling is summarized in
Figure 5.
Figure 5. Scheme of the Abeta pathway in inducing impaired neuronal insulin signaling.
9. Large-Size Proteins and AD
HSPs are a large class of proteins well known for playing a relevant role in the protein quality control (PQC) machinery [
167,
168,
169,
170]. HSPs are normally produced under physiological conditions but become upregulated under stress conditions. Their main function is to control the correct folding of nascent proteins and prevent the aggregation of protein misfolded forms. They are also involved in cell signaling and the transport of proteins across mitochondrial membranes. HSPs have been classified in several ways based of their size, intra or extracellular localization, mechanism of action, and dependence/independence on ATP of their activity. According to their molecular weight, six families have been distinguished: small HSP (sHSP) with molecular weight lower than 40 kDa, Hsp40, Hsp60, Hsp70, Hsp90 and Hsp100. Some HSPs are very specific in their activity, others are very general, and often they may work in tandem or operate in a sequential way. Based on the mechanism of action, they have been also grouped in holding, folding, and disaggregating HSPs [
171].
Holding HSPs bind partially folded proteins and maintain the substrates on their surfaces to await the availability of folding HSPs such as, for example, Hsp40 that “holds” client proteins to facilitate the folding action of Hsp70 [
172]. The group of holding HSPs comprises the family of sHSPs which are ATP-independent chaperones. They are characterized by the presence of a conserved alpha-crystalline domain that recognizes hydrophobic surfaces of client partially folded proteins [
173]. sHSPs are usually assembled in dynamic oligomeric structures that may easily undergo change in subunit composition for interacting with target proteins [
174].
Folding HSPs (Hsp60, Hsp70 and Hsp90) are real molecular machines that rely on conformational changes induced by ATP binding and hydrolysis to mediate the refolding/unfolding of their substrates [
167,
168,
169,
170,
171]. Albeit with different detailed mechanisms [
175,
176], folding HSPs recognize and interact with hydrophobic regions exposed by partially unfolded or misfolded proteins and use energy from ATP hydrolysis to stabilize folded conformations.
Disaggregating HSPs (e.g., Hsp104, Hsp105 and Hsp110) rely on ATP binding and hydrolysis to promote the solubilization of protein aggregates [
170,
171]. They belong to the AAA+ protein family (Adenosine Triphosphatases with diverse activities) as they share a common ATPase domain and structural organization in large ring-shaped complexes. They have been defined “Threading Machines” as they operate sequentially on consecutive small traits of aggregates [
177].
Due to their activity in regulating the correct cellular functionality, HSPs are deemed to be powerful therapeutic agents against neurodegenerative diseases [
178,
179,
180,
181,
182]. In fact, it has been proven that the overexpression of specific HSPs reduces the neurotoxicity of misfolded protein aggregates [
168,
171]. However, new results from in vivo and in vitro studies have demonstrated possible the negative influence of HSPs [
181,
183,
184]. It is generally accepted that HSPs do not interact with proteins in their monomeric functional form. Rather, HSPs are capable of interfering with different steps of the aggregation process [
185], working alone or in tandem or cascade. This, together with the still lacking clear recognition of which are the most dangerous species, makes a full comprehension of the various roles of HSPs extremely arduous.