Remarkably, the coexistence of ACS and cancer in the same patient strongly influences prognosis [
13]. The treatment in this setting is very challenging, and it should be patient-tailored [
14].
2. The Pathophysiologic Mechanism of Coronary Artery Disease in Cancer Treatment
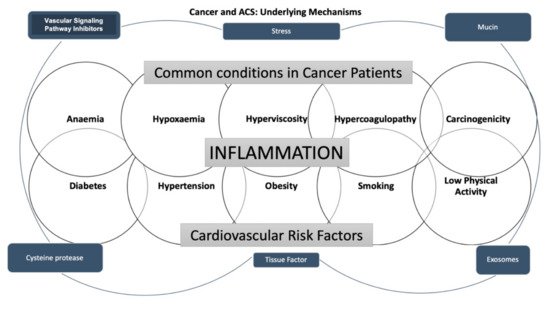
Cancer and heart diseases share cardiovascular (CV) risk factors, such as diabetes, hypertension, obesity, smoking, and low physical activity [
16]. In addition, some cancer-related conditions, such as anemia, hypoxemia, and hyperviscosity, are known to lead to ACS development because of an impaired balance between oxygen supply and consumption [
17].
On the other hand, it is acknowledged that malignant hypercoagulopathy occurs in cancer patients [
3]. It has been claimed that the cancer pro-coagulant factors released noticeably increase the thromboembolic risk [
18]. It is well recognized that vascular wall inflammation contributes to the pathogenesis of atherosclerosis [
19]. The interaction between monocytes, macrophages, and cancer cells is thought to be responsible for releasing tumor necrosis factor, interleukin-1, and interleukin-6 into the bloodstream, causing endothelial damage, which contributes to thrombosis [
20]. Furthermore, it also has been well assessed that pro-coagulant and tissue factors, such as k-Ras, vascular endothelial growth factor receptor, p53, phosphatase, tensin homolog, microparticles, and exosomes, are mainly secreted in tumor patients; in addition, coagulation factors such as VII, IX, X, and XIII also play an essential role in the thrombotic process [
21,
22]. Moreover, it is widely accepted that mucins, containing binding sites for P- and L-selectins, are involved in leukocytes, endothelial cells, and platelets activation. Consequently, the hemostatic system’s abnormal activation and regulation in malignancy patients plays an essential role in cancer progression and cardiovascular events [
23]. Finally, post-traumatic stress developed after a cancer diagnosis can be related to the increased risk of myocardial infarction [
7].
Hereafter, it is well accepted that in oncologic patients, not only the classic cardiovascular risk factors, but also malignancy-related factors such as cancer type and stage and therapeutic strategies play a complex role in ACS development. In addition, considering that symptoms might be masked by other conditions such as an advanced state of the disease, several co-morbidities, the analgesic effect of treatment against cancer pain, and neurotoxic effects of chemotherapy, a silent clinical presentation commonly occurs [
24].
The main mechanisms of cancer-induced CAD are shown in Figure 1.
Figure 1. The scheme summarizes the underlying mechanisms of the associations between cancer and acute coronary syndrome.
3. Epidemiology
Lung, prostate, gastric, pancreatic, and breast cancer have been established to be the most frequent types associated with ACS. The highest in-hospital mortality rates and major adverse cardiovascular and cerebrovascular complications (MACCEs) have been observed in lung cancer [
25]. The incidence of ACS in patients with newly diagnosed cancer is expected to be significatively higher in the first 6 months after the diagnosis and advanced cancer stages [
26,
27]. Indeed, its occurrence is almost two-times higher compared to the general population, with a cumulative incidence of myocardial infarction of 2.0% among cancer patients, compared to 0.7% in controls [
26].
Moreover, remarkably, in clinical practice, it must be carefully taken into consideration that patients who have neoplastic diseases, such as colon cancer, also carry a significantly higher bleeding risk [
26].
Finally, several concomitant conditions such as thrombocytosis, thrombocythemia, and anemia are associated with active lymphoma or leukemia patients that raise the incidence of ACS from 1.4 to 11.2% [
28].
4. Chemotherapy and ACS Risk
Androgen suppression therapy, used to treat prostate cancer, is associated with a higher incidence of metabolic syndrome and cardiac complications. In addition, fluoropyrimidines are the second-most-frequently reported cardiotoxic agents [
29], with an incidence of cardiotoxicity of 2–34% for 5-fluorouracil (5-FU) and 3–9% for capecitabine [
30,
31]. The most common manifestation is chest pain, which is sometimes atypical but which may be an expression of ACS [
32,
33].
An average time interval between the administration of 5-FU and the onset of cardiac symptoms of three days (range: 2–5 days) has been reported; this drug is frequently associated with electrocardiographic abnormalities such as ST-segment changes and T-wave inversion. The underlying mechanisms seem to be coronary vasospasm and thrombus formation; in addition, direct cellular damage to cardiomyocytes and endothelial cells is likely to occur and promotes platelet aggregation and thrombosis [
34]. Moreover, cisplatin may cause arterial thrombosis through endothelial cell dysfunction, thromboxane production, and an increment of von Willebrand factor activity platelet aggregation and activation [
35].
Vascular endothelial growth factor inhibitors (bevacizumab, sorafenib, and sunitinib) may induce cardiac ischemia and arterial thrombosis due to vasospasm, inflammation, and platelet activation. The mechanism appears to involve alterations in nitric oxide synthesis [
36]. The incidence of angina with these drugs varies from 1 to 15%.
Another group of Tyrosine-kinase inhibitors (TI), such as nilotinib and ponatinib, may cause endothelial apoptosis with increased factor VII levels, establishing a prothrombotic state [
37]. Cardiovascular events, including cerebrovascular accidents and peripheral vascular injuries, are pretty common with ponatinib [
26]. Additionally, a 25% increased risk of cardiovascular events is reported in women treated with aromatase inhibitors (anastrozole and letrozole). Similarly, the use of immunomodulatory agents such as lenalidomide, pomalidomide, and the proteasome inhibitor carfilzomib has also been associated with an increase in cardiovascular events, particularly ACS [
38]. Finally, the treatment with immune checkpoint inhibitors was also found to be related to an increased ACS risk, in addition to the most frequent complications, such as myocarditis. The activation of immune cells in coronary atherosclerotic plaques appears to contribute to the destabilization of atherosclerotic lesions, leading to plaque rupture and cardiovascular events [
39].
The main chemotherapy agent-related mechanisms of CAD and ACS are shown in Table 1.
Table 1. Mechanism of Antineoplastic Drug-Induced Cardiotoxicity.
|
Class
|
Agents
|
Cardiotoxic Effects
|
|
Immunomodulatory
|
Lenalidomide [40]
Pomalidomide [41]
Immune Checkpoint Inhibitors [42,43]
|
Endothelial Dysfunction → Destabilization of atherosclerotic lesions → Plaque rupture → Cardiovascular events
|
|
Anti-microtubule
|
Paclitaxel [44]
|
Vasoconstriction, Endothelial injury → Cardiovascular events
|
|
Proteasome Inhibitor
|
Carfilzomib [45]
Bortezomib [46]
|
Cardiac ubiquitin–proteasome dysfunction → Endothelial injury→ Cardiovascular events
|
|
Aromatase Inhibitors
|
Anastrozole [47]
Letrozole [47]
|
Vasoconstriction, Endothelial injury → Cardiovascular events
|
|
Anti-metabolites
|
5-fluorouracil (5-FU) [30]
Capecitabine [48]
Gemcitabine [49]
Nilotinib [50]
|
Coronary Vasospasm
Thrombus Formation
Direct cardiomyocytes and endothelial cells damage → Increase of Von Willebrand Factor’s activity → Cardiovascular events
|
|
BRC-ABL tyrosine kinase inhibitors
|
Nilotinib [50]
Ponatinib [51]
|
Coronary Atherosclerosis
Endothelial Apoptosis → Increase of Factor VII Levels → Prothrombrotic state → Cardiovascular events (cardiac, cerebrovascular, and peripheral events)
|
|
Vascular Endothelial Growth Factor Inhibitors
|
Bevacizumab [52]
Sorafenib [53]
Sunitinib [54]
Pazopanib [55]
Regorafenib [56]
Axitinib [57]
Ramucirumab [58]
Aflibercept [59]
|
Vasospasm
Inflammation
Platelet Activation → Cardiac ischemia and arterial thrombosis
|
|
Alkilating agents
|
Cyclophosphamide [60,61]
|
Endothelial dysfunction → platelet aggregation and activation → cardiovascular events
|
|
Vinca-alkaloids
|
Vincristine [62]
|
Thrombus Formation
Endothelial Injury → Cardiovascular Events
|
|
Platinum
|
Cisplatin [63]
|
Endothelial dysfunction → Thromboxane Production → Thrombus Formation → Platelet aggregation and activation → Cardiovascular Events
|
|
Anti-tumor antibiotics
|
Bleomycin [64]
|
Endothelial dysfunction → Platelet aggregation and activation → Cardiovascular Events
|
5. Radiotherapy and ACS
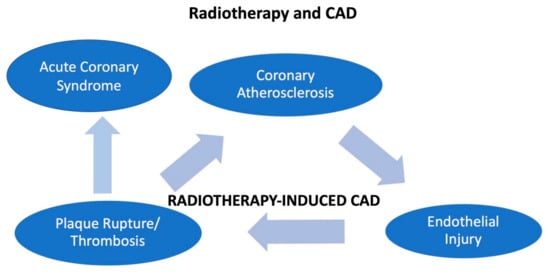
A direct endothelial injury can be induced by radiotherapy, since it has been well shown that a radiotherapy-related coronary artery disease (CAD) causes micro- and macrovascular progressive injuries. Involvement of the Ostia of the left main and right coronary artery is considered typical [
65], with a prevalence of 85%, and it is related to the radiation field [
66]. However, radiotherapy damages remain clinically silent for a long period after radiotherapy, therefore ACS is unlikely to occur during the treatment [
67].
A dose of 0.50 Gy is considered the cut-off for atherosclerosis risk [
68]. It has been noted that radiation produces an increase of myofibroblasts and macrophages, leading to intimal proliferation and causing a pro-thrombotic condition. Furthermore, atherosclerosis is expected to be enhanced by radiation-induced inflammation [
69]. On the other hand, radiation-induced myocardial fibrosis is involved in myocardial ischemia and consequently in myocardial dysfunction, with an incremental risk of myocardial infarction proportionally increasing with the radiation duration and the age at the time of exposition [
65].
In addition, even at low doses, radiations lead to microvascular damage, lowering the capillary bed’s density, reducing the vascular reserve. It has also been postulated that this damage enhances myocardial fibrosis, resulting in ischemia [
65].
The main mechanisms of radiotherapy-induced CAD are summarized in Figure 2.
Figure 2. The figure shows how radiotherapy may induce coronary disease and acute coronary syndrome in patients with cancer.
6. Clinical Presentation
In the general population, NSTEMI is the most common clinical presentation of ACS in cancer patients [
14,
70].
Significantly, NSTEMI could be due not only to CAD progression, but even to concomitant conditions, including the imbalance between O
2 supply and demand because of anemia and dehydration [
17].
Indeed, myocardial infarction with nonobstructive coronary arteries (MINOCA) and Takotsubo syndrome may also occur in patients with cancer, primarily women [
71].
Symptoms of ACS are generally atypical in malignancy patients, and less than one-third of them experience chest pain, and less than half have dyspnea. For this reason, careful clinical evaluation of patient history, the presence of risk factors, electrocardiogram findings, cardiac biomarkers, and echocardiographic imaging may allow ACS diagnosis in neoplastic patients [
72].
A further point to consider is that these patients receive fewer cardiovascular therapies and less frequently undergo invasive strategies [
2]. For patients with NSTEMI, evidence indicating a clear advantage of percutaneous revascularization treatment is scarce, especially in conditions of clinical stability, and this is due to the predisposition to bleeding, as reported by current guidelines [
5,
73,
74].
7. Management
There is no complete agreement on the most appropriate treatment of cancer patients due to a lack of data to guide clinicians towards the best-tailored treatment for these patients [
13].
Medical versus interventional management in cancer patients with NSTEMI should be chosen after carefully evaluating the risk/benefit ratio and the stability of the clinical status under medical treatment [
5,
74]. On the other hand, the approach to STEMI patients is like that of the general population [
75].
In patients with ACS, a multidisciplinary team should be in charge of choosing the best treatment between conservative or invasive strategies. Patients with cancer and ACS must be immediately admitted to intensive care and monitored.
7.1. Cancer and Percutaneous Coronary Intervention
Percutaneous coronary intervention (PCI) improves the survival rate of ACS patients, lowering early and late cardiac events. A U.S. study analyzed all individuals undergoing PCI between 2004 and 2014 in the Nationwide Inpatient Sample: 6,571,034 PCI procedures were included, and current and previous cancer rates were 1.8% and 5.8%, respectively [
76].
In the same registry, 18,052 patients had a diagnosis of lymphoma (0.25%) [
77], and 15,789 patients had a diagnosis of leukemia (0.24%) [
78].
In the real world, however, only a low percentage of patients with cancer and ACS perform PCI (from 54.2% for lung cancer to 70.6% for hematologic malignancies, vs. 82.3% for no cancer) [
79]. In the acute myocardial infarction in Switzerland (AMIS Plus) registry, cancer patients underwent PCI less frequently (OR 0.76; 95% CI 0.67–0.88) compared to no-cancer patients [
2]. Likely, patients with leukemia were less likely to undergo coronary angiography (48.5% vs. 64.5%) and PCI (28.2% vs. 42.9%) compared with those without leukemia [
80]. In a retrospective analysis from a non-academic center, coronary angiography was performed in only 47% of patients with cancer, while 53% were treated conservatively [
81]. Besides, in a single-center study, the median time to PCI was 10 h among the cancer patients and 7.5 h among the control group [
82].
The Society for Cardiovascular Angiography and Interventions expert consensus statement recommends, in cancer patients, radial access to reduce the risk of vascular complications, bleeding, and MACE. In contrast, the femoral approach should be reserved for complex coronary interventions, rotational atherectomy, or the need for intra-aortic balloon pumps [
86].
Regarding the devices employed, bare-metal stents have been used in the past, while now, third-generation drug-eluting stents are indicated for the lower risk of thrombosis and shorter duration of dual antiplatelet therapy (DAPT) [
87]. In addition, the use of a fractional flow reserve is advised to better assess the severity of coronary stenosis. In contrast, intravascular ultrasound and optical coherence tomography can be used to ensure optimal stent apposition and expansion [
86]. Current guidelines do not clearly state that invasive revascularization is not indicated in cancer. However, the European Society of Cardiology guidelines on NSTEMI suggest that an invasive strategy should be withheld in a subgroup with nonobstructive CAD and comorbidities such as cancer [
74].
Besides, patients with cancer had more comorbidities and were older.
Using a nationwide quality registry of all patients admitted for a first MI in Sweden, cancer was associated with major bleeding during a median follow-up of 4.3 years (HR 1.45; 95% CI 1.34–1.57) [
94].
Bleeding is related to the type and stage of cancer; the gastrointestinal tract tumors are at a greater risk [
95]. Kwok et al., in the US Nationwide Readmission Database, observed a higher 90-day readmission for bleeding after PCI in patients with active cancer (4.2% in colon, 1.5% in lung, 1.4% in prostate, 0.6% in breast, and 1.6% in all cancers) compared to 0.6% among patients with no cancer [
96]. In a U.S registry on PCI interventions in cancer patients, colon and prostate cancer were associated with bleeding risk (respectively, OR 3.65, 95% CI 3.07–4.35 and OR 1.41, 95% CI 1.20–1.65) [
76]. In the same registry, the diagnosis of Hodgkin Lymphoma was associated with increased odds of bleeding complications (OR 1.12, 95% CI 1.05 to 1.20), whereas these odds were not significantly associated with a non-Hodgkin’s diagnosis [
77].
Furthermore, a leukemia diagnosis was associated with significantly increased odds of in-hospital bleeding (OR: 1.87; 95% CI: 1.56–2.09) [
78].
After PCI, the active cancer group had clinically relevant bleeding during both DAPT and single antiplatelet therapy (SAPT) periods, and a multivariate Cox regression hazard analysis revealed cancer activity as a significant independent risk factor for bleeding (
p = 0.023) [
97].
A PCI registry analysis performed in Berna, including 13,000 patients, of which 10% had a historical diagnosis of cancer, found no difference in major ischemic events but an increased risk of major bleeding at one year [
87].
In the BleeMACS project, a multicenter observational registry enrolling patients with ACS undergoing PCI worldwide in 15 hospitals, after one year, patients with cancer more often experienced bleedings (6.5% vs. 3%,
p < 0.001), and in a multiple regression analysis, the presence of cancer was the strongest independent predictor for bleedings (HR 1.5, CI 1.1–2.1,
p = 0.015) [
6]. According to ESC NSTEMI guidelines, active malignancy (excluding non-melanoma skin cancer) within the past 12 months is a major bleeding risk factor [
74].
7.2. Pharmacological Treatment of ACS in Cancer Patients
In cancer patients with ACS, there is an increased risk of both thrombotic and hemorrhagic events [
93]. Therefore, the clinical decision on antiplatelet drugs and DAPT, aimed at mitigating the risk of stent thrombosis, is challenging for cardiologists and oncologists.
Observational data show that cancer patients with ACS were less likely to receive guideline-recommended drugs [
98].
A particular set of patients with cancer and ACS have thrombocytopenia. In a study on this population, subjects who did not receive ASA had a seven-day survival rate of 6% compared with 90% in those who had ASA (
p < 0.0001) without severe bleeding complications [
100].
In another retrospective study of cancer patients with chronic thrombocytopenia who underwent cardiac catheterization for ACS, aspirin therapy (alone or in combination with clopidogrel) was used in 66 patients (67.3%), whereas 27 patients (27.6%) were on dual antiplatelet therapy with a low incidence of bleeding complications and neither procedure-related antiplatelet nor therapy-related cerebrovascular events [
101]. A single-center prospective study in cancer patients with a recently placed (1–12 months) Drug-Eluting System (DES) demonstrated that Optical Coherence Tomography (OCT) imaging allows the identifying of low-risk cancer patients who may safely discontinue DAPT and proceed with cancer-related surgery or procedures [
101]. Finally, in the AMIS Plus registry, cancer patients received P2Y12 blockers (OR 0.82; 95% CI 0.71–0.94) and statins (OR 0.87; 95% CI 0.76–0.99) less frequently [
2].
7.3. Platelet Concentration Thresholds for Individual Elements of the Therapy
The current expert consensus recommendation by the Society for Cardiovascular Angiography and Interventions (SCAI) sets the lower level of platelet count for aspirin therapy at 10,000/µL and for DAPT (aspirin and clopidogrel) at 30,000/µL [
86]. In the catheterization laboratory, a reduced bolus of unfractionated heparin of 30–50 U/Kg is required in patients with platelets <50,000 µL [
86]. Platelet transfusions may be considered in thrombocytopenic patients who develop bleeding during or after cardiac catheterization or when there is a rapid drop in platelets or coagulation abnormalities or if the platelet count falls below 10,000/µL, whereas prophylactic platelet transfusion is not recommended unless required by the oncology/hematology team [
5,
13,
86].
If the platelet count falls below 30,000/µL, revascularization and DAPT should be decided after a preliminary multidisciplinary evaluation (interventional cardiology/oncology/hematology) and a risk/benefit analysis [
86]. Ticagrelor, prasugrel, and IIB-IIIA inhibitors should be avoided unless platelet counts are more than 50,000/mL [
13,
86].