Membrane vesicles, a group of nano- or microsized vesicles, can be internalized or interact with the recipient cells, depending on their parental cells, size, structure and content. Membrane vesicles fuse with the target cell membrane, or they bind to the receptors on the cell surface, to transfer special effects. Based on versatile features, they can modulate the functions of immune cells and therefore influence immune responses. In the field of tumor therapeutic applications, phospholipid-membrane-based nanovesicles attract increased interest. Academic institutions and industrial companies are putting in effort to design, modify and apply membrane vesicles as potential tumor vaccines contributing to tumor immunotherapy.
1. Introduction
Nanovesicles composed of lipid bilayers have aroused considerable interest and attention for fundamental study and practical applications. There are two types of phospholipid-membrane-based nanovesicles: pure lipid and/or protein vesicles and comparatively complex cell-membrane-derived vesicles (also called extracellular vesicles (EVs)) [
1]. Natural or synthetic lipid and/or protein ingredients make nanovesicles, involving liposomes or proteoliposomes, an ideal model of the membrane system with the advantages of an easy and low-cost production [
2]. Furthermore, cell-derived membrane vesicles are regarded as nano- to micrometer-sized containers comprising components such as cellular proteins, nucleic acids and lipids, for the reason that cell plasma or cytosol membranes can enclose these contents while membrane vesicles are secreted [
3].
Increasing studies have noticed that EVs are versatile communication tools to establish a link between tumor and host, contributing to tumor development, progression and metastasis. Both tumor and nontumor cells secrete lots of EVs, having a local impact on tumor cells, or traveling through blood vessels to bring about distant influence [
4]. Furthermore, bacterial membrane vesicles (BMVs), a type of cell-membrane-based EVs, which are derived from bacteria membrane architecture, own nanoscale vesicle structures containing biomembrane elements of phospholipids. Additionally, BMVs consist of considerable proteins, for example, original bacterial antigens and pathogen-associated molecular pattern components [
5]. Therefore, antigen-presenting cells (APCs) are able to recognize and absorb BMVs, and subsequently signaling pathways in immune cells are activated followed by specific immune responses. The molecular components and biological activity of BMVs or further-modified ones render them possible to be a potent vaccine to treat infectious or noninfectious diseases, including cancer [
6].
As membrane vesicles have a general characteristic, they contain a lipid bilayer structure that can package hydrophobic and hydrophilic compounds. Despite the practical condition that they are loaded with compounds from the parental cells, a further modification, transformation and fabrication could be conducted as needed to achieve expected goals, such as an enhanced output level, toxicity reduction, targeting improvement, etc. [
7]. So far, how membrane vesicles take a part in tumor vaccines is a hot topic.
2. Different Origins of Membrane-Based Nanovesicles Are Likely to Act as Tumor Vaccines
There are two primary types of phospholipid-membrane-based nanoparticles, including liposomes and extracellular vesicles (EVs). As EVs are a complex group of biomembrane-based nanostructures, based on their size, biogenesis and function, diverse classification methods have been used. According to the diameter of EVs, they could be roughly classified into small EVs (~100 nm) or large EVs (~100–1000 nm and/or >1000 nm) [
8]. Moreover, in terms of biogenesis, EVs can be precisely categorized into exosomes (30–100 nm) produced from the perinuclear luminal membrane and released through multivesicular fusion with the cell membrane, microvesicles (namely microparticles, 100–1000 nm) generated from the cell membrane budding and apoptotic bodies (500–2000 nm) produced through the protrusion of apoptotic cell membrane by dying cells [
3,
9,
10,
11]. On the basis of modification or not, natural EVs and engineered ones are included in the family of EVs. Furthermore, EVs have various origins, for example, generated from prokaryotic or eukaryotic cells, or from cell or plasma membranes, since a number of cells are able to secret EVs and subsequently EVs are filled with original components [
1].
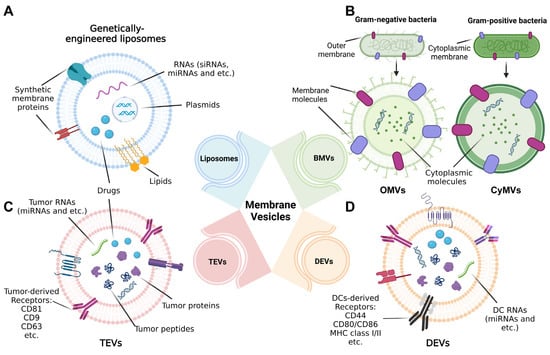
We then focus on the use of biomembrane-based nanoparticles as a kind of tumor vaccine in the field of tumor immunotherapy or precaution. The classification, structure and composition of four major types of these nanoparticles are going to be exemplified in the following sections, involving liposomes, BMVs, tumor-derived EVs (TEVs) and dendritic-cell-derived EVs (DEVs) (Figure 1).
Figure 1. Structure of nanoscale vesicles standing for tumor vaccines in the range of biomembrane-based nanovesicles. (A) Liposomes are genetically engineered with genes (including RNAs and plasmids) and proteins or modified with drugs or other small molecules. (B) For bacteria membrane vesicles (BMVs), outer membrane vesicles (OMVs) and cytoplasmic membrane vesicles (CyMVs) are, respectively, representatives of Gram-negative and Gram-positive bacteria-generated membrane vesicles, whose structures separately rely on the origins of bacteria. (C) Extracellular vesicles (EVs) derived from tumor cells (TEVs) and (D) Evs secreted by dendritic cells (DCs); namely, DEVs are typical cell-membrane-based vesicles widely used for tumor immunotherapy, and their composition depending on parental cells presents different forms, for example, types of membrane receptors.
2.1. Liposomes
From a biological point of view, since a cornucopia of lipids and proteins are combined together to naturally form cell membranes, in vitro natural lipids (for example phospholipids) or synthetic components are able to self-assemble into spherical bilayer nanoparticles, namely liposomes. Depending on the preparation methods, the size of the liposomes varies from each other ranging from small (3–5 nm) to giant vesicles (>1 μm) [
12].
In terms of the biomedical application of liposomes as a tumor vaccine, it is not surprising that simplified membrane vesicle liposomes alone could not perform effective vaccination in various diseases, since the structure and composition are limited and blood circulation times and stability lack satisfaction when they are applied. Thanks to its versatility, scientists take advantage of the properties of phospholipid membranes including chemical (amphiphilicity) and mechanical (stability, permeability and bending and stretching elasticity) peculiarity [
13,
14]. Therefore, the modification and functionalization of liposomes are necessary and available. Based on seminal reconstitution protocols, liposomes can achieve characteristics and be smart. For instance, liposomes could be stabilized by covering them with densely packed coats such as biocompatible PEG chains (poly (ethylene glycol)) and a crystalline bacterial cell surface layer [
15,
16]. In addition, according to the charge and specific membrane structure, reconstructed and engineered liposomes are present to mitigate drug delivery problems (safety, efficiency, internalization and targeting ability) [
2,
17]. Then, the liposome capacity of the efficiently delivering and suitably releasing cargoes (drugs, antigens, siRNA, etc.) can be improved. Taking some construction means as an example, Lian, Shu et al. designed cationic liposomes containing si-CD47 and si-PD-L1 and modified them with EpCAM (epithelial cell adhesion molecule), so they not only enhanced the immune therapeutic efficacy of liposomes, but also improved the targeting ability owing to the overexpression of EpCAM proteins in tumor cells [
18].
2.2. Bacterial Membrane Vesicles
BMVs are capable of transporting a variety of molecules (proteins, nucleic acids and toxins), and to some extent, the structure of the vesicles and composition of the cargoes differ between Gram-positive and -negative bacteria [
19,
20]. Both of them exhibit significant effects on innate and adaptive responses. For example, BMVs have been demonstrated to have protective characters against exogenous pathogens or endogenous mutants and have been applied for vaccine exploration [
21,
22].
For Gram-negative bacteria-derived BMVs, it is widely regarded that the vesicle structure is formed from three stratified layers of the fluid phospholipid bilayer, peptidoglycan cell wall and phospholipid membrane characteristically carrying lipopolysaccharide (LPS) in turn from inside to outside [
23]. As a critical participant in bacterial communication and homeostasis, outer membrane vesicles (OMVs) are naturally generated from Gram-negative bacteria, and thus contain pathogen-associated molecular patterns (PAMPs). Therefore, these vesicles, such as pathogen mimetic adjuvants, possess intrinsic immunostimulatory properties acting as a vaccine. Moreover, since membrane vesicles are capable of drug delivery, their natural composition could be enriched by modifying these vesicles with other immunomodulatory agents [
24,
25,
26].
In the aspect of the structure of Gram-positive bacteria-derived BMVs, depending on their own construction, these BMVs lack the package of the outer membrane, while they are coated with rigid peptidoglycan cell walls from the cytoplasmic membrane layer [
27]. Hence, these cytoplasmic membrane-generated BMVs are named CyMVs. Although the biological processes of Gram-positive BMVs are less understood than Gram-negative BMVs, they are still being taken into consideration for vaccine applications. The vaccination efficacy of BMVs from certain bacterial strains has been estimated as a strategy to fight against Gram-positive bacteria, and vaccine safety has been detected as well through monitoring toxin-specific antibodies. For the purpose of controlling and decreasing toxicity, approaches to producing genetically engineered BMVs emerge one after another. For instance, Wang, Xiaogang et al. purified BMVs from Staphylococcus aureus to establish an
S. aureus vaccine platform which could package cytosolic and secreted proteins, such as cytolysins and phenol-soluble modulins, contributing to BMVs biogenesis and detoxification [
28].
2.3. Tumor-Cell-Derived EVs
As EVs play a crucial role in cell–cell communication, tumor-cell-derived EVs (TEVs) take part in tumorous progression, tumor microenvironment modulation and distant metastasis, and even accelerate these processes [
29,
30].
Compared with liposomes, the contents and structure of EVs are relatively complicated. Meanwhile, the innate composition of TEVs inherited from parental tumor cells has gained a lot of attention. Considerable evidence from recent studies has demonstrated that there are lots of oncoproteins (such as phosphorylated epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), stromal-cell-derived factor 1 (SDF1), etc.) and oncogenic RNA (in particular miRNAs) in TEVs, making a contribution to tumor growth, invasion, migration and angiogenesis [
31,
32,
33,
34]. For these TEVs carrying parent-cell-specific signatures, they permit an interaction between the targeting cells and are potentially used to be explored or confirmed as biomarkers in liquid biopsies for distinctive diseases [
35]. Due to the existing biological qualities of TEVs, development for them acting as potential therapeutic strategies becomes suitable and overwhelming. For example, approaches inhibiting the preparation and secretion of native EVs or loading TEVs with antitumor drugs or RNAs which encode information of disrupting tumor progression are emerging rapidly [
36].
Since TEVs are assembled and packaged in a tumor-cell-specific manner, the components (in particular, the tumor antigens) are distinct from the EVs from mesenchymal stem cells or blood cells. Peptides in antigen-rich TEVs could be presented by major histocompatibility class (MHC) receptors and then cause the following interaction with immune cells (CD8
+, CD4
+ T cells or natural killer cells (NKs)) to help enhance cancer immunotherapy [
22,
37]. Additionally, to maintain biocompatibility and elevate the immunogenicity of TEVs, the formulation should be well designed, in order not only to guarantee biosecurity, but also to positively regulate antitumor immune responses and deliver therapeutics to tumor sites. In numerous basic or clinical studies, engineered or nonengineered, autologous or nonautologous TEVs have been fabricated and administrated to biomedical model species or human subjects [
38].
2.4. Dendritic-Cell-Derived EVs
Cell-membrane-based EVs are also secreted by immune cells in tumor tissues to contact the rest of the immune cells and tumor cells [
39]. Immune-cell-derived EVs play a crucial role in cell–cell communication for tumor cells to escape from immunological surveillance and for further studies to design potential tumor vaccines [
40]. Among these EVs, a relatively large focus has been put on dendritic cells (DCs)-derived EVs (DEVs or dexosomes) as a tumor vaccine, and thus DEVs have become an attractive candidate for tumor immunotherapies.
In a typical immune loop, antigen-presenting cells (APCs), among which DCs are the most potent APCs, prime the immune responses when the immune system defends against tumorous, viral or bacterial diseases [
41]. It is commonly believed that DCs expressing MHC class I or II molecules on the cell surface interact with other immune cells such as CD8
+ or CD4
+ T lymphocytes and natural killer cells to initiate an immune reaction [
42,
43]. During EVs secretion from DCs, it has been found that EVs also encapsulate peptide-MHC complexes (p-MHC) together with costimulatory molecules such as CD40 and CD86 [
44,
45,
46]. Additionally, it could carry abundant immunoregulatory cargos, including cytokines, complement factors and immunosuppressive or active molecular mediators [
47]. It has been verified that in the absence of APCs, DEVs own the ability to activate T cells since they can directly interact with T-cell receptor (TCR) complexes [
48]. Flourishing evidence proves that the capability of modified or engineered DEVs to elicit antigen-specific antitumor immune responses could be enhanced when costimulatory factors are upregulated and immunoregulatory/immunosuppressive signals are reduced [
49]. It is no wonder that DEVs continue to be a promising nanomaterial for further research on vaccination in tumor immunotherapy.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14112446