Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The co-torrefaction of several biomasses may be a viable solution in the study area, as it produces biofuels and addresses waste-treatment concerns. Furthermore, the parameters of co-torrefaction, including temperature, reaction time, mass yield, energy yield, and the composition of the H/C and O/C ratio of the co-torrefied materials, are similar to those for coal composition. Different reactor types, such as fixed-bed, fluidized-bed, microwave, and batch reactors, are used for co-torrefaction, in which biomass blends with optimized blend ratios.
- co-torrefaction
- reactors
- bioenergy
- biomass
1. Introduction
Since the beginning of the industrial era in the 18th century, the world has consumed most of the fossil fuels (such as coal, oil, and natural gas) at a high speed [1]. The widespread use of fossil fuels has lead to two major crises: energy depletion and global warming. As a result, the development of renewable energy and the reduction in carbon dioxide emissions have become a critical priority in the 21st century [2][3]. People in various countries depend on biomass as a sustainable energy source to meet the expanding energy demands and support economic growth [4][5]. Most biomasses have a low carbon content and high oxygen, hydrogen, and sulfur contents, which maximizes air pollution and greenhouse gas emissions [6][7]. In the opinion of most experts, the development of renewable energy, at present, is essential to reduce the use of fossil fuels, greenhouse gas emissions, and ecological pollution. Renewable biomass or bioenergy is the most abundant energy source in technologies to date [8][9][10][11].
Various categories of biomass resources are processed using various thermochemical techniques, such as torrefaction and pyrolysis, including gasification, which uses higher temperatures (≥200 °C) to valorize biomass into bio-solids and bio-oil, including syngas [10][12][13]. Renewable energy generated from wind, solar, hydro, geothermal, and biomass [14][15] sources is replacing energy derived from fossil fuels. Bioenergy derived from biomass has some potential to partially replace non-renewable sources, such as coal (electricity generation). However, compared to coal, biomass, by nature, has a lower energy density, greater moisture levels, and volatiles [16]. Due to this, biomass must undergo pre-treatment to enhance its qualities before it can be used instead of fossil fuel [17]. Pyrolysis, which depends on temperature and heating rate, promotes the synthesis of bio-solid, bio-oil, and syngas from biomass resources in an inert environment [18][19]. Torrefaction uses moderate temperatures (200–300 °C) to convert biomass into fuel bio-solids [20][21][22]. Co-torrefaction is the heating of two biomasses at 200–300 °C in an inert environment. Due to the higher biomass-to-coal ratio, fuel has greater flexibility and produces less tar [23][24].
Several previous studies emphasized co-thermal processing, such as the co-pyrolysis of waste resins and conventional biomass, and also reported their interaction effects. For example, the synergistic impact on liquid and gas yields was identified when biomass was combined [25]. As a result of the addition of pine cones to polymeric materials, the number of gaseous products increased more than expected, resulting in a lower char yield [26]. Co-torrefaction is feasible for the production of bio-solids [27]. The bio-solid fuels can be utilized for co-firing or environmental remediation applications through the thermochemical process (torrefaction) [28]. The use of biomass for co-torrefaction with a low calorific value implies the maximum amount of oxygen and hydrogen in the biomass. Co-torrefaction can increase the calorific value of a bio-solid fuel by removing the moisture content and the decomposing part of the volatile matter [29]. Microwave co-torrefaction of an empty fruit bunch with used engine oil at 300 °C has been shown to improve the high heating value (28.0 MJ/kg) of solid fuel [30]. Numerous types of biomass waste can be used without harming the environment [31]. Solid biomass and bio-oil can be combined by mixing several biomass ratios, thus reducing waste disposal and greenhouse gas emissions [32][33][34]. The co-torrefaction of various feedstocks improves the fuel properties of the product [35]. It is challenging to store hygroscopic raw biomass because of its higher moisture content and lower energy density [36][37]. This means that the use of raw biomass as a fossil fuel alternative, such as coal, is limited because of these features. Processing, on the other hand, can address the drawbacks of raw biomass. To achieve this, biomass can be pretreated by a process known as co-torrefaction. Temperatures of 200 °C–300 °C are used under vacuum, and nitrogen is supplied during the heating of raw biomass [22][36]. Furthermore, the study observed that co-torrefaction can significantly increase the properties of biomass at some levels [38], such as reducing the moisture content of the raw biomass, resulting in higher energy density and a higher heating value (HHV) [39]. Furthermore, the hygroscopic characteristic of raw biomass has been transformed into hydrophobic fuel [40]. Figure 1a presents the total publications obtained from different countries, while Figure 1b indicates the dynamics of the yearly publications.
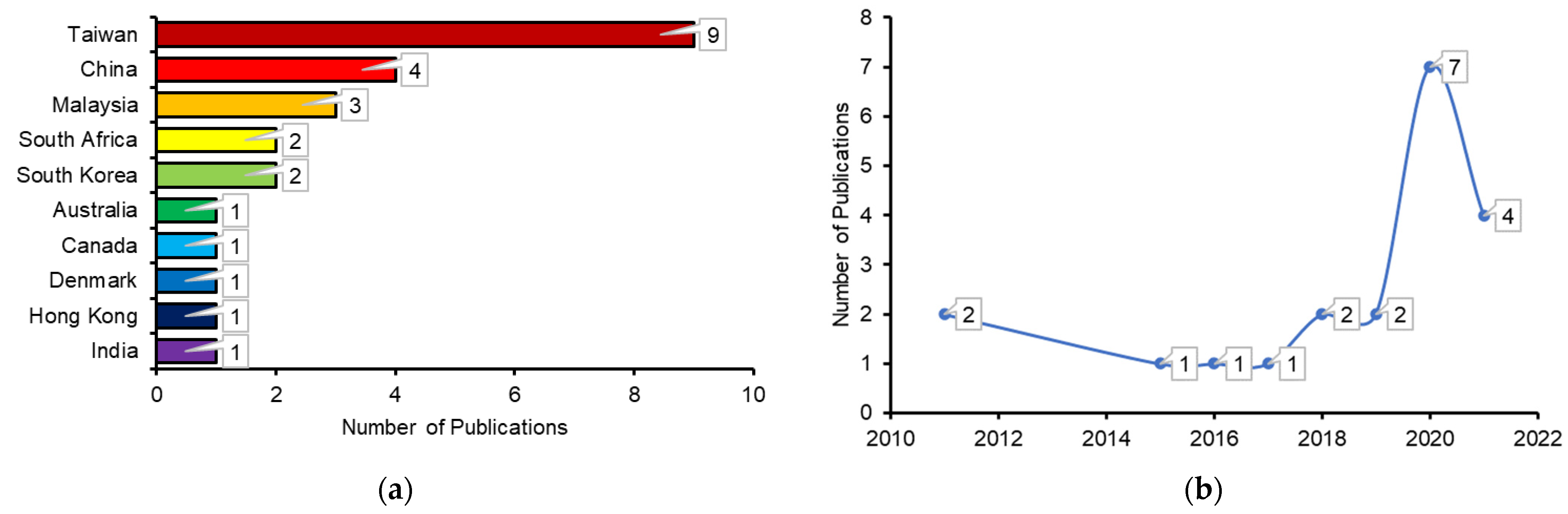
Figure 1. Number of publications in the co-torrefaction process (a) country-wise for the whole period and (b) year-wise retrieved from the Scopus database (2 October 2022).
The following table summarizes the most recent research conducted on the co-torrefaction of biomass and garbage, including studies, features, and outcomes. The co-torrefaction of biomass as feedstock did not indicate a synergetic effect. The torrefied product presented an inconsequential improvement in HHV for use as a fuel co-fire [41]. The co-torrefaction process used empty fruit bunch (EFB) pellets as the primary feedstock and cooking oil (UCO) as the secondary feedstock to enhance the calorific value, and hence the increased quality of the EFB pellets. As a result, high-calorific-value torrefied pellets that are more environmentally friendly were produced. Microwave co-torrefaction (MCT) is a new technology that combines microwave heating with co-torrefaction [42]. Torrefied biomass pellets were compared with typical furnace-based co-torrefaction in terms of their properties, manufacturing process, waste reduction, and energy-conversion efficiency [42]. A torrefied biomass can be integrated into coal-fired boilers through direct, indirect, or simultaneous co-firing systems [43]. The study shows that microwave heating is an innovative technology integrated with the torrefaction process [42][44]. The work conducted on the co-torrefaction of various biomass and waste materials is summarized in Table 1.
Table 1. Latest developments in the co-torrefaction process.
| Sr. No. | Biomass Type | Blending Ratio | Process and Type of Reactor | Process Condition | Outcome | Application | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Waste epoxy resin and fir | Mixing ratio of fir:waste epoxy resin is 1:3 | Co-torrefaction Conventional heating batch-type reactor |
Temperature: 120 °C–180 °C, time: 10 min–40 min |
Solid yield 76.86%. Enhancement in HHV 1.12 Energy yield 85.79% Improved evaporation of volatile compounds. Solid yield adversely affected |
Improvement of biochar | [23] |
| 2 | Sewage sludge and Leucaena | Mixing ratio of sewage sludge:Leucaena is (75:25%) | Co-torrefaction Microwave heating |
Microwave power level 100 W, time: 30 min, temperature: 170 °C–390 °C |
Bio-char made from pure Leucaena wood has a CO2 adsorption capacity of 53 mg/g | Solves waste-water problem. Production of biofuels |
[44] |
| 3 | Biomass and coal | Blending ratio of biomass:coal is (30:70%) | Vertical tubular furnace | Temperature: 300 °C, time: 60 min |
Produced mass yield: (57.0–63.8%), energy yield: (77.0–89.0%), (18.1–22.2%) reduction in CO2 emissions | Enhances the quality of coal | [45] |
| 4 | Microalgae and Lignocellulosic biomass | - | Co-torrefaction A gas chromatographic furnace with a glass reactor |
Temperature: 250 °C, time: 60 min |
Better temperatures (92.6%) result in higher energy efficiency, but the moisture content of the feed mixture quickly decreases this efficiency (16.9 to 57.3% for 70% moisture) | High production of bio-char with high calorific value | [35] |
| 5 | Mango seed and passion shell with optoelectronic sludge | Blending optoelectronic sludge with mango seed in a 25/75 ratio | Wet co-torrefaction Microwave reactor |
Temperature from 120 °C to 180 °C), reaction duration from 10–40 min | Higher heating value of 19.0 MJ/kg, 92.1% of energy yield, fuel ratios of 1.60–1.82, and an energy return on investment of 14.7% |
The production of fuel of the highest grade | [46] |
| 6 | Food sludge and lignocellulosic biowaste | Mixing macadamia husk and sludge in a (25/75%) ratio (db%) | Wet co-torrefaction Microwave reactor |
Temperature: 150 °C, duration: 20 min |
HHV:19.6 MJ/kg; decreased ash content; first-order kinetics; increased thermal stability and combustion efficiency of biochar; 7.4 energy return on investment; 45.2% reduction in carbon gas emissions | Production of bio-solid and nutrient recovery | [41] |
| 7 | Empty fruit bunch pellet, used cooking oil, and waste engine oil | - | Co-torrefaction Microwave reactor |
Temperature: 200, 250 °C and 300 °C, heating rate: 50–65 °C/min, time: 5–8 min | There is an 85.5 wt% mass yield Fuel ratio: 1.8. Carbon content: 68.3%. Fixed carbon: 62.3%. HHV: 28.0 MJ/kg. |
Production of solid fuel with greater improvement | [30] |
| 10 | Hemicellulose, cellulose, lignin, xylan, dextran, xylose, and glucose | Weight ratio (1:1:1) |
Co-torrefaction Conventional heating thermogravimetry |
Temperature: 230 °C, 260 °C and 290 °C | There is no synergistic effect of co-torrefaction on weight loss of the blend | - | [47] |
| 11 | Textile sludge and lignocellulose biowaste (macadamia husk) | - | Wet co-torrefaction | Temperature: 120 °C–180 °C, time: 10–30 min | Amount of fixed carbon: 29.8%, HHV: 19.7 MJ/kg |
Production of biofuel | [41] |
| 12 | Mango branches (MBr), waste newspaper (Np), and low-density polyethylene (LDPE) | Three binary mixtures prepared, with a mass ratio of 1:1 | Bench-scale tubular reactor |
Temperature: 300 °C | (MBr-LDPE) carbon content: 71.94% HHV: 35.84 MJ/kg |
Improved fuel characteristics that allow co-firing | [48] |
| 13 | Food sludge and six widely produced lignocellulose bi-wastes | Blending ratios of 0/100, 25/75, 50/50, and 100/0 | Microwave heating system | Torrefaction temperature (120, 150, and 180 °C), reaction time (10, 20, and 30 min) | Food sludge blended with macadamia husk (25/75 db%) highest fixed carbon content (25%) HHV: (19.6 MJ/kg) | Renewable energy resource. | [41] |
2. Co-Torrefaction Mechanism and Operation Parameters
2.1. Co-Torrefaction Process
Co-torrefaction occurs when two biomass blends undergo a process and are converted into a bio-solid. Figure 2 illustrates the process of co-torrefaction. In Figure 2, two residual/waste biomass were blended together in various blending ratios (0:100, 25:75, and 50:50%). The feedstock was placed into the furnace for pre-treatment (co-torrefaction) and run under vacuum with a supply of nitrogen, so that an inert atmosphere was created in the furnace in order to avoid the possible ignition of the sample. The duration of this step depended on the flow rate and size of the furnace. After purging the supply of nitrogen was interrupted, the sample was kept in a crucible placed in the central furnace with a heating rate of 10 °C/min in the temperature range of 200–300 °C for a residence time of 30 min to 2 h [49]. Three products (bio-oil, bio-solid, and bio-gas) were obtained from the furnace following the co-torrefaction of blending the residual biomass. The main outcome of the co-torrefaction process is a high-quality bio-solid product [50]. The co-torrefaction process is endothermic at low temperatures, but progresses toward an exothermic process when char is formed during the thermal degradation of lignocellulosic biomass at high temperatures [51]. During the degradation of the components, various reactions are involved. The first stage is to remove the moisture content at 110 °C. The following stage is to remove inbound moisture or a fully moisture-free environment when the temperature increases to 200 °C. At 200 °C, the torrefaction process begins to decompose volatile matter to produce solid, liquid, and gaseous products. At 200–250 °C, the stage of decomposition of hemicellulose occurs that is characterized by limited devolatilization, and a solid structure is formed. During this stage, C-C, C-O, and inter- and intramolecular hydrogen breakdowns occur, which form condensable liquids and non-condensable gases. The stage of 250–300 °C is the extensive part of the torrefaction process in which hemicellulose decomposes into volatiles and solid products are formed [42].
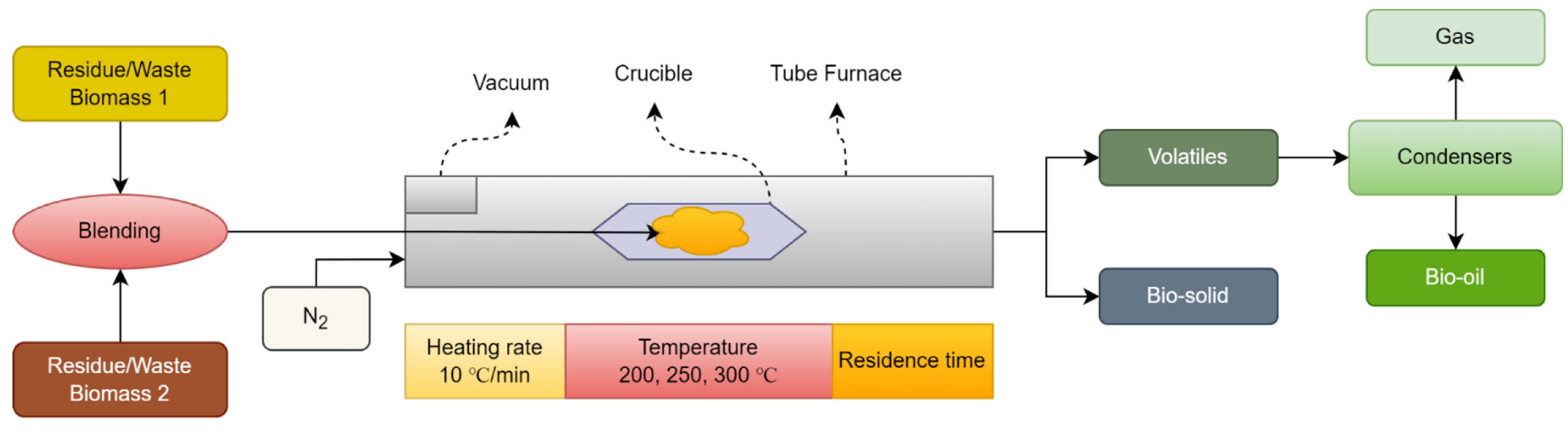
Figure 2. Co-torrefaction process.
2.2. Synergistic Effect
When two or more biomass wastes combine to generate a more significant impact than either of them could produce alone, this is called a synergistic effect. When materials are combined, synergistic effects may be used to increase co-torrefaction yields. Furthermore, the combination of OS with MIse and Passiflora edulis shell (PEsh) for wet torrefaction (WT) has a synergistic impact on the increase in HHV content in co-torrefied bio-solid, especially in a 75/25% ratio [46]. As a consequence of these results, combining OS with fruit bio-waste is an additional effective way to help the process, involving the betterment of the bio-solid as a product. Therefore, it is likely to be used instead of traditional fuels in the future (e.g., coal) [52].
2.3. Operating Parameters
The co-torrefaction process utilized a variety of biomasses that were thermochemically processed and acquired desirable qualities. During the co-torrefaction of biomass, numerous operating parameters affected the co-torrefaction process, such as the role of temperature, residence duration on mass and energy yields, and the HHV of biomass, and the Van Krevelen diagram.
2.3.1. Studying the Role of Temperature and Residence Time on Mass and Energy Yields
The mass and energy yields of the co-torrefied biomass varied with temperature and the reaction time. The increase in temperature and residence time decreased the mass and energy yields, while the energy density increased. The mass yield of OS decreased when the co-torrefaction temperature increased from 120 °C to 180 °C, from 98.4% after 10 min at 150 °C to 79.9% after 30 min at 180 °C. The main constituents of raw sewage (such as low-molecular-weight hydrocarbons) were degraded with the increasing co-torrefaction intensity. This reaction had an energy density of 1.14 and a 100% energy yield at 150 °C for a reaction time of 30 min [46]. During co-torrefaction at a temperature of 150 °C and a reaction time of 10 min, a further 99.4% energy yield was obtained with an associated energy density of 1.01 [46]. As a result, unnecessary energy consumption is reduced, and a high HHV of bio-solid is obtained [46]. During 20 min of torrefaction at 150 °C, 95.2% of the energy was extracted, with a maximum energy density of 1.20.
The mass and energy yields were affected by various blend ratios and types of biowaste used [29]. The OS and bio-waste co-torrefied together produced more than 80% of the total mass and energy yield. These were the same yields reported for the microwave-based torrefaction of OS, which may have been due to the heating of the samples from the inside at lower temperatures and for shorter periods, leading to their higher energy efficiency [53]. This might be because microwave-irradiation heating modes are more energy efficient, as they can heat the interiors of materials at lower co-torrefaction temperatures for shorter periods of time [54]. The bulk bio-solid yields decreased when the ratio of the OS/biowaste blend was reduced from 75/25 to 25/75%, especially in the case of the PEsh OS blend [46]. This phenomenon occurs because biomass has a higher microwave-absorption capacity than sludge, resulting in the considerable devolatilization of biomass as the percentage of bio-waste in the mix increases [55]. Because MIse and PEsh have lower energy densities than those of pure OS, they increase the energy density of pure OS. When OS is combined with MIse and PEsh bio-wastes, the energy and mass yields are the same. When the OS/bio-waste blending ratio was altered from 75/25% to 25/75%, the bio-solid mass yields decreased from 95.1% to 92.1% for OS combined with MIse and 93.4–65.2% for OS mixed with PEsh. These results are consistent with the other research investigating the co-torrefaction of sewage sludge and Leucaena using microwave heating [54]. Note that when the OS–PEsh and OS–MIse co-torrefied bio-char was mixed at 50/50 and 25/75%, the mass recovery and energy yields of the OS–PEsh co-torrefied bio-solid were substantially lower than those of the OS–MIse co-torrefied bio-solid [46]. Furthermore, when bio-waste and sludge are mixed for co-torrefaction, heat can degrade a significant amount of hemicellulose and cellulose, reducing the mass and energy yields of bio-char while maintaining its higher energy content [55]. Furthermore, bio-solids produced from co-torrefied food waste offer an improved substitute for peat in terms of their thermal qualities when combined with sugarcane bagasse, rice straw, Pisdium guajava, Annona squamosal, macadamia husk, and pistachio husk, respectively [41].
2.3.2. Studying the Role of Temperature and Residence Time on HHV
The quality of a bio-solid can be significantly influenced by the proportions of biomass used in the mixing process. The combination of OS with MIse and PEsh bio-waste generates a bio-solid with different HHVs. The Mlse and PEsh biowastes were observed to have experimental HHVs of 19.4 and 18.6 MJ/kg, respectively, which was significantly higher than OS (15.5 MJ/kg) after 30 min of torrefaction at 150 °C; microwave-assisted WT was used to mix textile sludge and lignocellulose bio-waste, and bio-char HHV increased in the same proportion as the blending ratios of the two types of bio-waste increased. Following 30 min of torrefaction at 150 °C, it was revealed that the maximum high-heating values of OS mixed with MIse and PEsh were better than those obtained with the other blending ratios (75/25 and 50/50%). These were 19.0 and 18.3 MJ/kg, respectively [46]. The resulting bio-solid had a maximum of 19.2–21.1 MJ/kg HHV, which was an increase over lignite coal (19.2–21.1 MJ/kg) [56]. A total of 55% of the carbon in bio-char was fixed carbon compared to raw food sludge (FS). The fixed carbon and ash contents of biomass increased when the FS and bio-waste were mixed. As a result, agricultural bio-waste can be appropriately disposed of by reusing it as renewable energy [57]. As the ratio of blending for bio-waste increased, the HHVs increased more than the FS; the energy density of the subsequent bio-solid also increased. Sewage sludge and Leucaena co-torrefaction produced a similar outcome. When bio-solid was created from torrefied food scraps, it had a significantly higher HHV than bio-solid created from torrefied food scraps alone (19.2–20 MJ/kg). The HHV content of FS with MH (25/75% dry basis) presented the highest amount of investigational HHV [41].
This is consistent with those previously described for torrefied wood and agricultural biomass after hydrothermal carbonization. The higher degree of carbonization of torrefaction significantly accelerated cellulose and hemicellulose degradation, resulting in a reduction in smoke (from fly ash, COx, NOx, and SOx) produced during biofuel combustion [58]. The increase in the temperature and reaction time of torrefaction steadily increases the HHV. The result is affected more by the reactor time of co-torrefaction than by the temperature of the OS bio-solid. The heating value of the bio-solid was 24.1 MJ/kg, at a temperature of 300 °C at a residence time of 45 min. The blended FS bio-solid had a maximum heating value of 18.9 MJ/kg at 150 °C at 30 min. The HHV of bio-solid from FS was 21.7% higher than that of the raw sludge [41]. The increase in torrefaction temperature decreased the mass yield from 84.2% (120 °C for 30 min) to 67.7% (200 °C for 30 min). It could be associated with protein breakdown and polysaccharides in solids of sludge [59].
2.3.3. Van Krevelen Diagram
The Van Krevelen diagram was first used to categorize the coal and estimate the compositional change throughout maturity by plotting O/C against H/C. In order to better understand fuel quality, one must consider the atomic ratios of the constituting elements. The HHV of biomass, for example, ranged from approximately 20.5 to 15 MJ/kg as the oxygen–carbon ratio increased from 0.86 to 1.03 [60].
The Van Krevelen diagram also compares torrefied and untorrefied biomass. Torrefied biomass has a higher carbon content and decreases oxygen and hydrogen contents compared to untorrefied biomass. The other aspect is that co-torrefied biomass has lower oxygen-to-carbon and hydrogen-to-carbon ratios compared to untorrefied biomass, as presented in Figure 3. Untorrefied biomass, such as OS 100%, MIse 100%, EFB 100%, Cv 100%, and Lc 100%, have low HHVs due to the higher O/C and H/C ratios, and co-torrefied biomass, such as OS:MIse (25:75%) torrefied = 150 °C, EFB with used UCO torrefied = 300 °C, Lc 50% torrefied = 300 °C, and Lc 100% torrefied = 300 °C, have high HHVs due to the low O/C ratio, as depicted in Figure 3. This discussion shows that torrefied biomass has better fuel than untorrefied biomass.
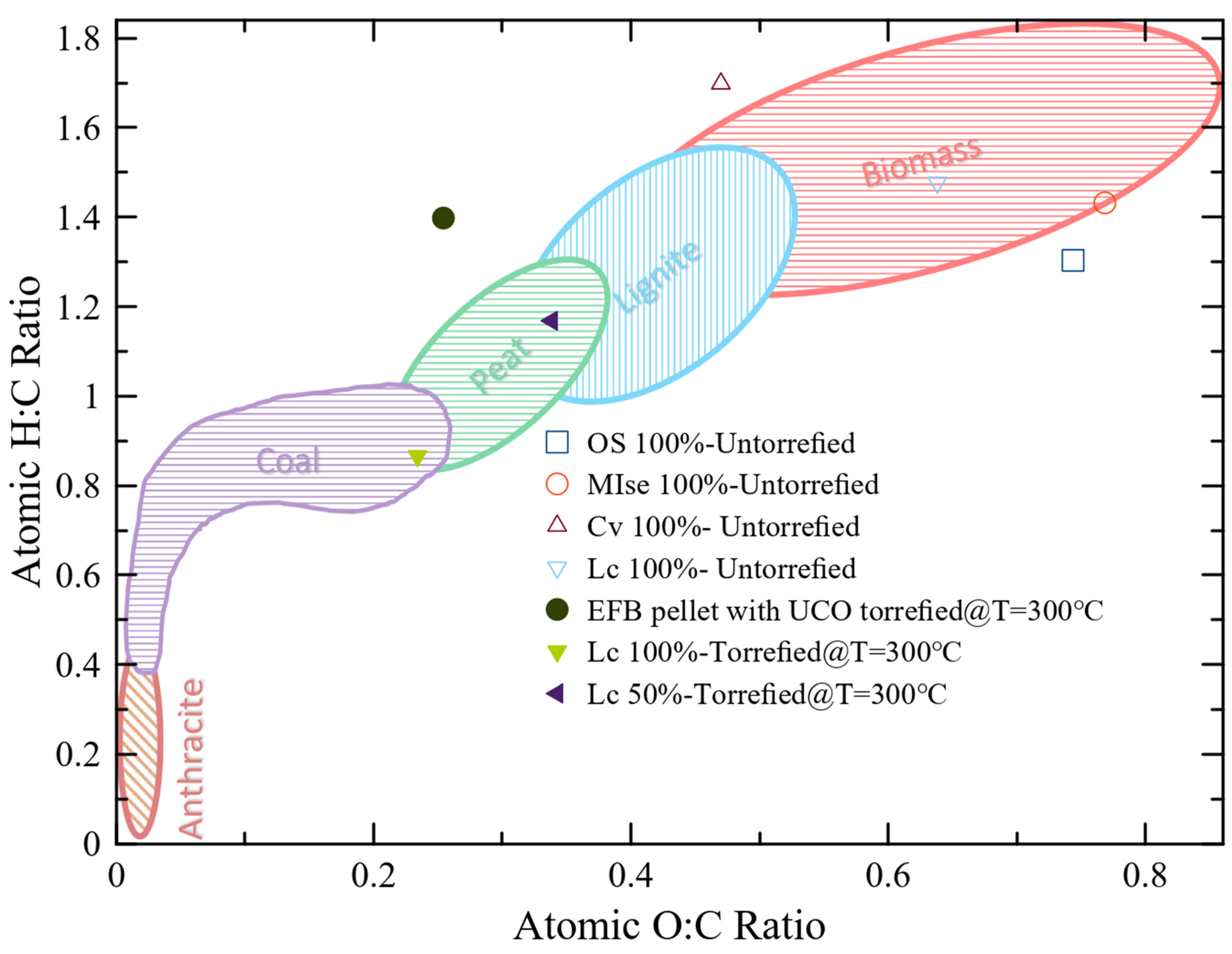
Figure 3. Van Krevelen diagram redrawn using the data from [61].
Figure 3 also presents the O/C and H/C values of the coal. Anthracite has elaborated low values of the O/C and H/C ratios and presents high-solid-fuel properties. After comparing the torrefied with untorrefied biomass in different literature surveys, it can be observed that the un-torrefied biomass outlies the coal value of the O/C and H/C ratios. However, the ratios of H/C and O/C of the torrefied biomass are close to those for coal. For example, EFB pellets with UCO, T = 300 °C show that the O/C- and H/C-ratio values are very similar to anthracite coal, showing that this biomass has a good fuel quality.
Fuel quality decreases as the O/C and H/C ratios increase. A decrease in the O/C ratio was compared to the raw materials Cv and Lc in bio-solids formed at 200 °C and 225 °C, indicating some deoxygenation. However, no structural alterations were observed because the H/C ratio was equivalent to the feedstock. Thermal processes, such as bio-solid torrefaction, results in O/C and H/C ratios compared to peat, lignite, and anthracite coal, further demonstrating the impact of temperature on fuel quality [29]. A temperature-dependent decrease in the H/C ratio was also observed at co-torrefaction temperatures higher than 250 °C. This indicates that the carbonaceous structure is reorganized as more aromatic compounds are produced [62]. The lignocellulosic structure of the bio-solid undergoes an enhanced rearrangement under high-torrefaction conditions, altering the porosity of the material by eliminating OH-binding groups [63]. Compared to raw biomasses, bio-solids also have a higher energy content due to their lower moisture content [61]. The calorific value of co-torrefied biomasses depends on the oxygen, hydrogen, and carbon content present in the feed. The feed contains a significant amount of oxygen and a low carbon content, so its calorific value is low and vice versa [64]. The primary objective of pre-treatment is to improve the carbon content and reduce the oxygen level. Co-torrefaction is used to reduce the oxygen concentration of biomass, which directly affects the heating value of any fuel and presents a higher calorific value. As a result, it is challenging to convert biomass into liquid fuels with an improved heating value. Products can be produced from the biomass of high-oxygen or -hydrogen contents [65].
This entry is adapted from the peer-reviewed paper 10.3390/en15218297
References
- Wang, T.; Zhai, Y.; Li, H.; Zhu, Y.; Li, S.; Peng, C.; Wang, B.; Wang, Z.; Xi, Y.; Wang, S.; et al. Co-hydrothermal carbonization of food waste-woody biomass blend towards biofuel pellets production. Bioresour. Technol. 2018, 267, 371–377.
- Zhu, Z.; Si, B.; Lu, J.; Watson, J.; Zhang, Y.; Liu, Z. Elemental migration and characterization of products during hydrothermal liquefaction of cornstalk. Bioresour. Technol. 2017, 243, 9–16.
- Abdalla, M.E.; Abdalla, S.A.; Taqvi, S.A.; Naqvi, S.R.; Chen, W.-H. Investigation of Biomass Integrated Air Gasification Regenerative Gas Turbine Power Plants. Energies 2022, 15, 741.
- Ge, S.; Foong, S.Y.; Ma, N.L.; Liew, R.K.; Mahari, W.A.W.; Xia, C.; Yek, P.N.Y.; Peng, W.; Nam, W.L.; Lim, X.Y.; et al. Vacuum pyrolysis incorporating microwave heating and base mixture modification: An integrated approach to transform biowaste into eco-friendly bioenergy products. Renew. Sustain. Energy Rev. 2020, 127, 109871.
- Naqvi, S.R.; Taqvi, S.A.A.; Mehran, M.T.; Khoja, A.H.; Naqvi, M.; Bokhari, A.; Saidina Amin, N.A. Chapter 2-Catalytic pyrolysis of biomass using shape-selective zeolites for bio-oil enhancement. In Bioenergy Resources and Technologies; Azad, A.K., Khan, M.M.K., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 39–60.
- Zafar, M.W.; Shahbaz, M.; Hou, F.; Sinha, A. From nonrenewable to renewable energy and its impact on economic growth: The role of research & development expenditures in Asia-Pacific Economic Cooperation countries. J. Clean. Prod. 2019, 212, 1166–1178.
- Qayyum, M.; Khoja, A.H.; Naqvi, S.R.; Ejaz, H.; Nawar, A.; Ansari, A.A. Development of Cost-Effective Fertilizer-Based Media for the Microalgae Cultivation Aimed at Effective Biomass Production. NUST J. Eng. Sci. 2020, 13, 45–51.
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691.
- Chen, W.-H.; Farooq, W.; Shahbaz, M.; Naqvi, S.R.; Ali, I.; Al-Ansari, T.; Saidina Amin, N.A. Current status of biohydrogen production from lignocellulosic biomass, technical challenges and commercial potential through pyrolysis process. Energy 2021, 226, 120433.
- khan, M.; Raza Naqvi, S.; Ullah, Z.; Ali Ammar Taqvi, S.; Nouman Aslam Khan, M.; Farooq, W.; Taqi Mehran, M.; Juchelková, D.; Štěpanec, L. Applications of machine learning in thermochemical conversion of biomass-A review. Fuel 2023, 332, 126055.
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764.
- Cheng, Y.W.; Chong, C.C.; Lee, S.P.; Lim, J.W.; Wu, T.Y.; Cheng, C.K. Syngas from palm oil mill effluent (POME) steam reforming over lanthanum cobaltite: Effects of net-basicity. Renew. Energy 2020, 148, 349–362.
- Farooq, W.; Ali, I.; Raza Naqvi, S.; Sajid, M.; Abbas Khan, H.; Adamu, S. Evolved Gas Analysis and Kinetics of Catalytic and Non-Catalytic Pyrolysis of Microalgae Chlorella sp. Biomass With Ni/θ-Al2O3 Catalyst via Thermogravimetric Analysis. Front. Energy Res. 2021, 9, 775037.
- Moriarty, P.; Honnery, D. Global Renew. Energy resources and use in 2050. In Managing Global Warming; Elsevier: Amsterdam, The Netherlands, 2019; pp. 221–235.
- Raza, M.; Inayat, A.; Ahmed, A.; Jamil, F.; Ghenai, C.; Naqvi, S.R.; Shanableh, A.; Ayoub, M.; Waris, A.; Park, Y.-K. Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing. Sustainability 2021, 13, 11061.
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Understanding the chemical and structural transformations of lignin macromolecule during torrefaction. Appl. Energy 2014, 121, 1–9.
- Mamvura, T.A.; Danha, G. Biomass torrefaction as an emerging technology to aid in energy production. Heliyon 2020, 6, e03531.
- Mahari, W.A.W.; Chong, C.T.; Lam, W.H.; Anuar, T.N.S.T.; Ma, N.L.; Ibrahim, M.D.; Lam, S.S. Microwave co-pyrolysis of waste polyolefins and waste cooking oil: Influence of N2 atmosphere versus vacuum environment. Energy Convers. Manag. 2018, 171, 1292–1301.
- Lam, S.S.; Mahari, W.A.W.; Jusoh, A.; Chong, C.T.; Lee, C.L.; Chase, H.A. Pyrolysis using microwave absorbents as reaction bed: An improved approach to transform used frying oil into biofuel product with desirable properties. J. Clean. Prod. 2017, 147, 263–272.
- Uemura, Y.; Sellappah, V.; Trinh, T.H.; Hassan, S.; Tanoue, K.-I. Torrefaction of empty fruit bunches under biomass combustion gas atmosphere. Bioresour. Technol. 2017, 243, 107–117.
- Atabani, A.E.; Pugazhendhi, A.; Almomani, F.; Rene, E.R.; Naqvi, S.R. Recent advances in the thermochemical transformation of biomass to bio-oil, biochar and syngas and its upgrading methods. Process Saf. Environ. Prot. 2022, 168, 624–625.
- Khan, A.A.; Gul, J.; Naqvi, S.R.; Ali, I.; Farooq, W.; Liaqat, R.; AlMohamadi, H.; Štěpanec, L.; Juchelková, D. Recent progress in microalgae-derived biochar for the treatment of textile industry wastewater. Chemosphere 2022, 306, 135565.
- Zheng, N.-Y.; Lee, M.; Lin, Y.-L. Co-processing textile sludge and lignocellulose biowaste for biofuel production through microwave-assisted wet torrefaction. J. Clean. Prod. 2020, 268, 122200.
- Inayat, M.; Shahbaz, M.; Naqvi, S.R.; Sulaiman, S.A. Chapter 3-Advance strategies for tar elimination from biomass gasification techniques. In Bioenergy Resources and Technologies; Azad, A.K., Khan, M.M.K., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 61–88.
- Sajdak, M. Impact of plastic blends on the product yield from co-pyrolysis of lignin-rich materials. J. Anal. Appl. Pyrolysis 2017, 124, 415–425.
- Wu, W.; Qiu, K. Vacuum co-pyrolysis of Chinese fir sawdust and waste printed circuit boards. Part I: Influence of mass ratio of reactants. J. Anal. Appl. Pyrolysis 2014, 105, 252–261.
- Wang, L.; Barta-Rajnai, E.; Skreiberg, Ø.; Khalil, R.; Czégény, Z.; Jakab, E.; Barta, Z.; Grønli, M. Effect of torrefaction on physiochemical characteristics and grindability of stem wood, stump and bark. Appl. Energy 2018, 227, 137–148.
- Yek, P.N.Y.; Mahari, W.A.W.; Kong, S.H.; Foong, S.Y.; Peng, W.; Ting, H.; Liew, R.K.; Xia, C.; Sonne, C.; Tabatabaei, M.; et al. Pilot-scale co-processing of lignocellulosic biomass, algae, shellfish waste via thermochemical approach: Recent progress and future directions. Bioresour. Technol. 2022, 347, 126687.
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887.
- Lam, S.S.; Tsang, Y.F.; Yek, P.N.Y.; Liew, R.K.; Osman, M.S.; Peng, W.; Lee, W.H.; Park, Y.-K. Co-processing of oil palm waste and waste oil via microwave co-torrefaction: A waste reduction approach for producing solid fuel product with improved properties. Process Saf. Environ. Prot. 2019, 128, 30–35.
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 13–28.
- Mehdi, R.; Khoja, A.H.; Naqvi, S.R.; Gao, N.; Amin, N.A. A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications. Catalysts 2022, 12, 798.
- Khan, S.A.; Ali, I.; Naqvi, S.R.; Li, K.; Mehran, M.T.; Khoja, A.H.; Alarabi, A.A.; Atabani, A.E. Investigation of slow pyrolysis mechanism and kinetic modeling of Scenedesmus quadricauda biomass. J. Anal. Appl. Pyrolysis 2021, 158, 105149.
- Madhu, P.; Vidhya, L.; Vinodha, S.; Wilson, S.; Sekar, S.; Patil, P.P.; Kaliappan, S.; Prabhakar, S. Co-pyrolysis of Hardwood Combined with Industrial Pressed Oil Cake and Agricultural Residues for Enhanced Bio-Oil Production. J. Chem. 2022, 2022, 9884766.
- Viegas, C.; Nobre, C.; Correia, R.; Gouveia, L.; Gonçalves, M. Optimization of Biochar Production by Co-Torrefaction of Microalgae and Lignocellulosic Biomass Using Response Surface Methodology. Energies 2021, 14, 7330.
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737.
- Sharma, H.B.; Dubey, B.K. Binderless fuel pellets from hydrothermal carbonization of municipal yard waste: Effect of severity factor on the hydrochar pellets properties. J. Clean. Prod. 2020, 277, 124295.
- Chen, W.-H.; Kuo, P.-C. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586.
- Yan, W.; Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Thermal pretreatment of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2009, 28, 435–440.
- Kethobile, E.; Ketlogetswe, C.; Gandure, J. Torrefaction of non-oil Jatropha curcas L.(Jatropha) biomass for solid fuel. Heliyon 2020, 6, e05657.
- Zheng, N.-Y.; Lee, M.; Lin, Y.-L.; Samannan, B. Microwave-assisted wet co-torrefaction of food sludge and lignocellulose biowaste for biochar production and nutrient recovery. Process Saf. Environ. Prot. 2020, 144, 273–283.
- Yek, P.N.Y.; Chen, X.; Peng, W.; Liew, R.K.; Cheng, C.K.; Sonne, C.; Sii, H.S.; Lam, S.S. Microwave co-torrefaction of waste oil and biomass pellets for simultaneous recovery of waste and co-firing fuel. Renew. Sustain. Energy Rev. 2021, 152, 111699.
- Li, J.; Brzdekiewicz, A.; Yang, W.; Blasiak, W. Co-firing based on biomass torrefaction in a pulverized coal boiler with aim of 100% fuel switching. Appl. Energy 2012, 99, 344–354.
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. CO2 adsorption on biochar from co-torrefaction of sewage sludge and leucaena wood using microwave heating. Energy Procedia 2019, 158, 4435–4440.
- Rizkiana, J.; Zahra, A.; Wulandari, W.; Saputra, W.; Andrayukti, R.; Sianipar, A.; Sasongko, D. Effects of Coal and Biomass Types towards the Quality of Hybrid Coal Produced via Co-Torrefaction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 823, 012028.
- Lin, Y.-L.; Zheng, N.-Y. Biowaste-to-biochar through microwave-assisted wet co-torrefaction of blending mango seed and passion shell with optoelectronic sludge. Energy 2021, 225, 120213.
- Liu, X.; Lin, Q.; Yan, Y.; Peng, F.; Sun, R.; Ren, J. Hemicellulose from plant biomass in medical and pharmaceutical application: A critical review. Curr. Med. Chem. 2019, 26, 2430–2455.
- Rago, Y.P.; Collard, F.-X.; Görgens, J.F.; Surroop, D.; Mohee, R. Torrefaction of biomass and plastic from municipal solid waste streams and their blends: Evaluation of interactive effects. Fuel 2020, 277, 118089.
- Hidayat, W.; Rubiyanti, T.; Sulistio, Y.; Iryani, D.A.; Haryanto, A.; Amrul, A.; Yoo, J.; Kim, S.; Lee, S.; Hasanudin, U. Effects of Torrefaction Using COMB Dryer/Pyrolizer on the Properties of Rubberwood (Hevea brasiliensis) and Jabon (Anthocephalus cadamba) Pellets. 2021. Available online: http://repository.lppm.unila.ac.id/id/eprint/32920 (accessed on 5 October 2022).
- Cheong, K.Y.; Kong, S.H.; Liew, R.K.; Wong, C.C.; Wong, C.S.; Ngu, H.J.; Yek, P.N.Y. Integration of microwave co-torrefaction with helical lift for pellet fuel production. Green Process. Synth. 2022, 11, 404–410.
- Chen, W.-H.; Kuo, P.-C.; Liu, S.-H.; Wu, W. Thermal characterization of oil palm fiber and eucalyptus in torrefaction. Energy 2014, 71, 40–48.
- Khan, S.R.; Zeeshan, M.; Masood, A. Enhancement of hydrocarbons production through co-pyrolysis of acid-treated biomass and waste tire in a fixed bed reactor. Waste Manag. 2020, 106, 21–31.
- Motasemi, F.; Afzal, M.T. A review on the microwave-assisted pyrolysis technique. Renew. Sustain. Energy Rev. 2013, 28, 317–330.
- Huang, Y.-F.; Sung, H.-T.; Chiueh, P.-T.; Lo, S.-L. Co-torrefaction of sewage sludge and leucaena by using microwave heating. Energy 2016, 116, 1–7.
- Huang, Y.-F.; Sung, H.-T.; Chiueh, P.-T.; Lo, S.-L. Microwave torrefaction of sewage sludge and leucaena. J. Taiwan Inst. Chem. Eng. 2017, 70, 236–243.
- Tian, H.; Jiao, H.; Cai, J.; Wang, J.; Yang, Y.; Bridgwater, A.V. Co-pyrolysis of Miscanthus Sacchariflorus and coals: A systematic study on the synergies in thermal decomposition, kinetics and vapour phase products. Fuel 2020, 262, 116603.
- Assad Munawar, M.; Hussain Khoja, A.; Hassan, M.; Liaquat, R.; Raza Naqvi, S.; Taqi Mehran, M.; Abdullah, A.; Saleem, F. Biomass ash characterization, fusion analysis and its application in catalytic decomposition of methane. Fuel 2021, 285, 119107.
- Raza, J.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Shakir, S.; Liaquat, R.; Tahir, M.; Ali, G. Methane decomposition for hydrogen production over biomass fly ash-based CeO2 nanowires promoted cobalt catalyst. J. Environ. Chem. Eng. 2021, 9, 105816.
- Tang, B.; Feng, X.; Huang, S.; Bin, L.; Fu, F.; Yang, K. Variation in rheological characteristics and microcosmic composition of the sewage sludge after microwave irradiation. J. Clean. Prod. 2017, 148, 537–544.
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018.
- Mehdi, R.; Raza, N.; Naqvi, S.R.; Khoja, A.H.; Mehran, M.T.; Farooq, M.; Tran, K.-Q. A comparative assessment of solid fuel pellets production from torrefied agro-residues and their blends. J. Anal. Appl. Pyrolysis 2021, 156, 105125.
- Cao, Y.; He, M.; Dutta, S.; Luo, G.; Zhang, S.; Tsang, D.C. Hydrothermal carbonization and liquefaction for sustainable production of hydrochar and aromatics. Renew. Sustain. Energy Rev. 2021, 152, 111722.
- Feng, Y.; Qiu, K.; Zhang, Z.; Li, C.; Rahman, M.M.; Cai, J. Distributed activation energy model for lignocellulosic biomass torrefaction kinetics with combined heating program. Energy 2022, 239, 122228.
- Colantoni, A.; Paris, E.; Bianchini, L.; Ferri, S.; Marcantonio, V.; Carnevale, M.; Palma, A.; Civitarese, V.; Gallucci, F. Spent coffee ground characterization, pelletization test and emissions assessment in the combustion process. Sci. Rep. 2021, 11, 5119.
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809.
This entry is offline, you can click here to edit this entry!
