大黄是从传统中药大黄中分离出的蒽醌的单体成分。它具有抗炎、抗氧化、抗细胞凋亡、抗菌等药理活性,以及保肾作用。大莱茵主要通过降低降血糖和降血脂,发挥抗炎,抗氧化和抗纤维化作用以及调节药物转运蛋白来发挥其肾保护作用。
1. Introduction
Rhein (molecular formula C15H8O6), a lipophilic anthraquinone, is the main component of Senna alexandrina Mill., Rheum palmatum L., Aloe barbadensis Miller, and Polygonum multiflorum Thunb [17]. It contains two hydroxyl groups and one carboxyl group and has strong polarity and electrochemical REDOX properties [18]. Rhein has a lot of pharmacological effects, such as anti-inflammation [19], anti-cancer [20], anti-fibrosis [21], antioxidation [22], hepatoprotective [23], nephroprotective [24], lipid-lowering [25], and antimicrobial activities [26]. In spite of this, its poor solubility and low bioavailability limit its clinical applications. The study of rhein and its derivatives has been enriched by advances in drug separation and synthesis. Diacerein is one of the most common and representative derivatives of rhein, and it is used for the treatment of arthritis owing to its ability in reducing osteoclast formation and inhibiting the synthesis of resorptive factors [27]. Additionally, nanodrug delivery systems have been designed to overcome the poor solubility of rhein [28]. The pharmacological effects of these compounds lay the groundwork for the treatment of liver disease, osteoarthritis, diabetes, atherosclerosis, and a variety of cancers [29,30,31,32,33]. However, it has recently been reported that rhein also causes hepatotoxicity and nephrotoxicity [22,34].
2. Nephroprotective Effect
The pharmacological benefits of rhein for human health are increasingly recognized. Rhein, and its derivatives, analogs, and compound preparations show the nephroprotective activities against various kidney disease, especially diabetic nephropathy (DN) and drug-induced AKI. One of the most common microvascular complications of diabetes is DN [
35], which is a leading cause of CKD and end-stage renal disease (ESRD) worldwide [
36]. The pathophysiology of DN is quite complex with two main initiating factors: abnormal metabolism and hemodynamic activity [
37]. Pathogenesis of DN includes metabolic dysregulation, inflammation, oxidative stress, abnormal cytokines, etc., [
38]. Considering multiple pathogenic mechanisms, we therefore need to develop drugs with multiple targets to treat DN effectively. The pathogenesis of AKI may be related to the specific injury of renal vessels, glomeruli, renal tubules, or interstitial compartments [
2]. AKI can cause apoptosis, autophagy, and regulated and genetically controlled cell death as a result of cell damage [
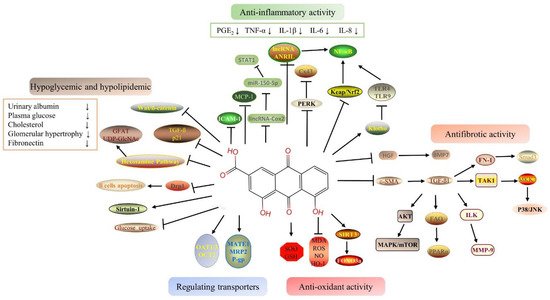
39]. Rhein has a variety of therapeutic targets as a TCM ingredient (or element), and its protective effect on the above targets has been gradually examined. Rhein is highly effective for the treatment of kidney-related diseases among natural components. The schematic mechanism of the signal pathway of the nephroprotective effects of rhein is shown in
Figure 1.
Figure 1. Signal pathway of the nephropropective effects of rhein.
2.1. Hypoglycemic and Hypolipidemic Proprieties of Rhein
Some studies have shown that the mitochondrial event chain caused by hyperglycemia may be one of the pathogeneses of diabetic nephropathy [
78]. Rhein treatment showed significant improvements in glucose-dependent and independent insulin secretion in db/db mice by preserving β-cell mass and inhibiting apoptosis [
45]. Another study [
47] showed that rhein protects pancreatic cells from apoptosis by inhibiting the expression of dynamin-related protein 1 (Drp1). It may be possible to prevent or treat diabetes by rhein in the near future. Rhein has been shown to improve insulin resistance and renal injury in rats and db/db mice [
79]. Furthermore, rhein and benazepril were evaluated individually and in combination in diabetic mice. Benazepril and rhein showed similar kidney protection [
40]. In db/db mice, a combined application had a better kidney protection effect [
40]. This reduced urinary albumin excretion, decreased plasma glucose, cholesterol, relieved glomerular hypertrophy, mesangial expansion and proliferation, and inhibited the expression of fibronectin (FN) and transforming growth factor- β1 (TGF-β1) [
40]. In order to elucidate the therapeutic mechanism of rhein on DN, Zheng J et al. (2008) examined the effect of rhein on the hexosamine pathway [
41]. Due to its role as a nutrient sensor, the hexosamine pathway has been associated with metabolic disorders and cellular hypertrophy when glucose levels are high [
80]. The hexosamine pathway is one of the mechanisms responsible for renal damage in diabetes. To mimic mesangial cells under diabetic conditions, transgenic mesangial cell lines (MCGT1) overexpressing glucose transporter 1 (GLUT1) were used. GLUT1, an integral membrane protein, transports glucose into mesangial cells via glucose gradients [
81].
2.2. Anti-Inflammatory Proprieties of Rhein
As a result of hyperglycemia, advanced glycation end products (AGEs) damage the vascular endothelium, glomeruli, and tubules, which facilitates the emergence of DN eventually [
85]. Diabetes is an entity of inflammation because its pathogenesis involves multiple inflammatory/proinflammatory factors, and it is characterized by chronic low-grade inflammatory disease [
86]. The model of early diabetic nephropathy was established and showed increases in serum microalbuminuria, NADPH oxidase expression, and PKR-like eukaryotic initiation factor 2α kinase (PERK) level, and a decrease in connexin 43 (Cx43) in renal tissue. Upregulation of nuclear transcription factors, such as PERK, reveals the presence of endoplasmic reticulum stress (ER stress) [
87]. Cx43, a family of connexins, may play a key role in exchanging small molecules within glomeruli and tubules in the kidney, which is essential for normal renal function [
43]. Argirein, a derivative of rhein, can be produced by combining rhein with L-arginine by forming a hydrogen bond. It has anti-inflammatory activity derived from rhein. Argirein (200, 100, and 50 mg/kg) is more effective than aminoguanidine (AMG) (100 mg/kg), which has anti-inflammatory activity as a positive reference agent in reversing the above changes [
43].
The mortality rate of AKI is about 70–80 % [
95,
96]. Currently, renal replacement therapy (RRT) is the only effective treatment for AKI [
97]. Inflammatory response inherent to sepsis is thought to be the direct mechanism of AKI [
98]. Based on rhein’s anti-inflammatory pharmacological activity, Yu C et al. (2015) explored the effects of rhein on sepsis-induced AKI by using lipopolysaccharide (LPS) and cecal ligation and puncture (CLP) models [
55]. The potential mechanism of rhein may be related to its anti-inflammatory effects. Rhein inhibited the activation of NF-κB via restraining the expression and phosphorylation of the relevant proteins in the NF-κB signal pathway and hindering the transcription of NF-κB p65 [
55]. It brings a new research direction for solving AKI related to endotoxemia. At the same time, the research of Liu M et al. (2021) also confirms this point [
73]. The 5/6 nephrectomy model (5/6 Nx) in rats and LPS-induced HK-2 cells were used in this study, and the results indicated that rhein inhibits inflammatory signaling pathways via decreasing the production of TNF-α, IL-6, and monocyte chemotactic protein (MCP-1) [
73]. In addition, by inhibiting NF-κB phosphorylation, rhein diminished LPS-induced NF-κB activation [
73]. The above results clearly indicate that rhein can be a promising therapeutic agent for renal disease by inhibiting multiple inflammatory mediators [
55,
73].
2.3. Antioxidant Proprieties of Rhein
Rhein attenuated APAP-induced hepatotoxicity and nephrotoxicity in a dose-dependent manner [
23]. The levels of serum glutamate-pyruvate transaminase (GPT), glutamate-oxaloacetic transaminase (GOT), urea nitrogen (UREA), creatinine (Crea), and reactive oxygen species (ROS) production were significantly decreased, and the contents of nitric oxide (NO), MDA, and GSH were recovered in the rhein treatment group [
23]. Rhein relieved APAP-induced liver and kidney injury by ameliorating oxidative stress [
23]. Diacerein (DIA) is used to treat osteoarthritis. DIA enters the body and is rapidly converted into its active metabolite rhein [
63]. Furthermore, studies have been conducted one after another on whether DIA can alleviate AKI. The antioxidant effects of DIA were reported to protect renal function against doxorubicin-induced AKI [
63].
2.4. Antifibrotic Proprieties of Rhein
CKD and chronic kidney failure (CRF) develop as a result of renal fibrosis. The pathological mechanism of renal fibrosis is relatively complex. There is a variety of stimulating factors or mediators, such as growth factors, cytokines and toxins, which induce the occurrence of fibrosis through a variety of mechanisms and signal pathways [
113,
114]. TGF-β has been proven to be a major pathogenic factor for the progressive development of renal fibrosis [
115]. TGF-β can induce renal tubular epithelial cells to transform into renal mesenchymal fibroblasts through the epithelial–mesenchymal transition (EMT) process [
116]. In addition to the TGF-β pathway, the Notch, Wnt, and Hedgehog signaling pathways can also be activated in response to renal injury, thereby promoting renal fibrosis [
117].
TGF-β regulates renal fibrosis progression through classic and non-classic pathways. The classical TGF-β pathway includes two signaling pathways, namely TGF-β/Smad and bone morphogenetic protein (BMP) [
115]. However, they have opposite effects although they have similar downstream Smad signaling pathways. Smad3 is a key downstream mediator of TGF-β signal transduction [
115]. BMP7 is a member of the TGF-β superfamily that antagonizes the effects of TGF-β [
118]. Like liver growth factor (HGF), they both have anti-fibrotic effects [
118]. Combined therapy of rhein and Danshensu (DSS) had a certain renal protective effect on chronic kidney damage. The mechanism may be related to anti-inflammatory and anti-fibrosis by downregulating the NF-κB-related pathway and inhibiting the TGF-β/Smad3 pathway, respectively [
56]. Other studies have shown that rhein improved renal function and reduced renal fibrosis and interstitial inflammation by inducing HGF and BMP7 production [
50].
In addition to the classical Smad signaling pathway, TGF-β can also regulate the downstream cellular response through other non-classical pathways and then adjust the pathological process of renal fibrosis [
115]. p38 mitogen-activated protein kinase (MAPK) is one of the atypical signaling pathways of TGF-β1. TGF-β1 activates the downstream signaling pathway MKK3-p38 MAPK cascade through the activation of TAK1, ultimately leading to cell fibrosis [
119]. Rhubarb and Astragalus capsules (RAC) that contain 2.25 mg/g rhein have been used in a clinical treatment for chronic kidney disease [
70].
2.5. Benefits of Rhein Via Drug-Transporter
Renal transporters transport endogenous substances, poisons, and drugs from the blood to the urine. As a result of renal injury, uptake transporters and efflux transporters are altered, which affects toxic excretion and aggravates renal injury [
133]. Previous research by our group has shown that the expressions of organic anion transporter 1 (OAT1), OAT3, and multidrug resistance related protein 2 (MRP2) were significantly decreased after cisplatin-induced AKI, which reduced the excretion of endotoxin and aggravated renal injury [
133]. There are few studies on the relationship between the various pharmacological effects of rhein and transporters. Zhu Y et al. (2022) [
24] showed that the gene levels and protein expressions of the renal transporters including Oat1, Oat3, Organic cation transporter 2 (OCT2), mammal multidrug, and toxin extrusion proteins 1 (Mate 1), Mrp2, and P-glycoprotein (P-gp) in vancomycin-induced nephrotoxicity (VIN) were significantly decreased. Plasma creatinine, BUN, and plasma indoxyl sulfate were not excreted efficiently. Rhein reversed the expressions of the above transporters, and thereby promoted the excretion of endotoxins and finally alleviated renal injury [
24]. The discovery of VIN’s pathogenesis expands the field of study on the kidney protection effects of VIN.
3. Toxicological Effects in Kidney
Total rhubarb anthraquinones (TRAs) include emodin, rhein, chrysophanol, aloe emodin, and other substances [
134]. However, the clinical cases of liver injury and the progression of kidney disease caused by TCM contained TRAs are increasing. Rhein is an important ingredient in TRAs, which affects the kidneys in a positive and negative way. Studies on the nephrotoxicity effects of rhein and the related mechanisms are shown in
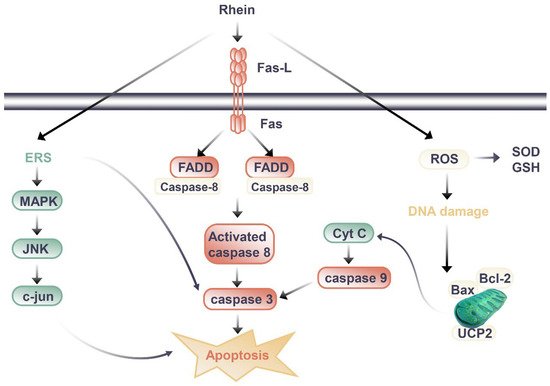
Figure 2.
图2.大黄萎缩肾毒性作用的信号通路。
大黄仁的肾脏保护和肾毒性作用之间的差异可能与大黄萎缩的剂量和持续时间有关。当大黄仁发挥保肾作用时,动物实验中大黄素的用量多为20-150mg/kg/d,给药时间多小于14天,当大黄素剂量略低时,最长时间为8周。在小鼠中,HU Y等人(2019)观察到长期给予大黄蛋白后肾毒性[
139]。将小鼠随机分为3组:空白组、低剂量大黄蛋白组(0.175g/kg)和高剂量大黄蛋白组(0.35g/kg)。该药通过管饲法给药60日[
139]。与同性别空白组相比,给药组小鼠的BUN和SCr水平升高,大剂量组小鼠体重下降[
139]。给药组雄性小鼠肾指数明显下降,GSH-Px含量降低,大黄蛋白大剂量组雄性小鼠TGF-β1表达升高[
139]。其潜在的毒性机制可能是由谷胱甘肽抗氧化系统的失衡引起的,谷胱甘肽抗氧化系统可诱导过度氧化、炎症反应和半胱天冬酶-3活化诱导的细胞凋亡[
139]。
为了合理、安全地使用莱茵,我们可能应该采取一些措施。一方面,如上所述控制大黄耸素给药的剂量和持续时间很重要;另一方面,中药的相容性可以增强其保护作用,降低毒性。例如,不同剂量的黄芪甲苷(10、20和40μM)可以减少大黄芸酶诱导的空泡、细胞融合和HK-2细胞坏死细胞的增加[
141]。在HK-2细胞中用大黄芪甲苷合用48 h后,细胞抑制率和LDH泄漏率均显著降低[
141]。相容性显著提高了细胞中SOD和GSH的含量,并下调了MDA的表达,这表明黄芪甲苷可以显著抑制大黄芪素引起的氧化应激损伤,进而保护细胞[
141]。
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196572