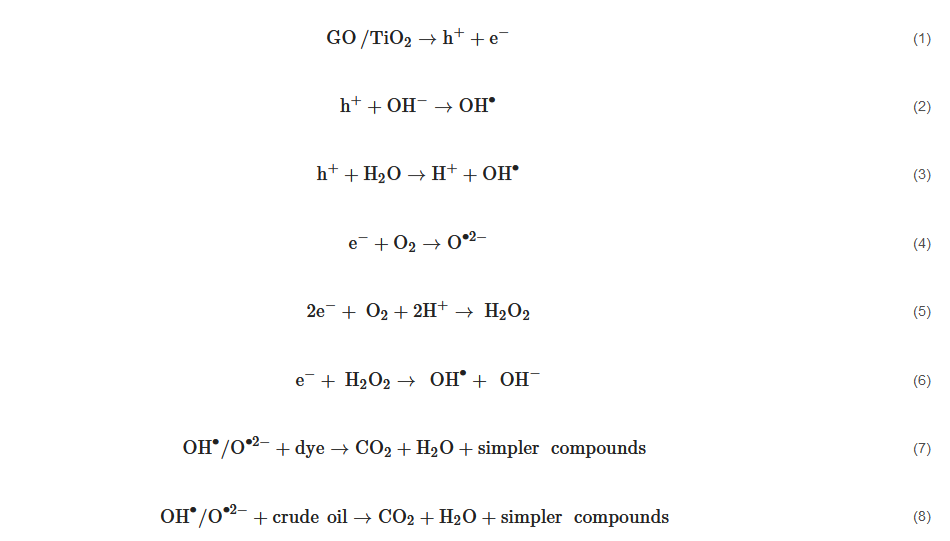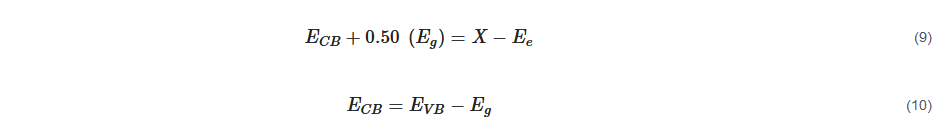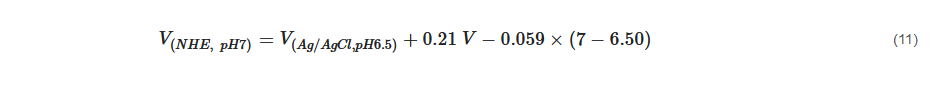Photocatalysis is a more recently applied concept and is proven to be able to completely remove and degrade pollutants into simpler organic compounds. Titanium dioxide (TiO2) is a fine example of a photocatalyst owing to its cost-effectiveness and superb efficiency. However, issues such as the high recombination rate of photogenerated electrons along with positive holes while being only limited to UV irradiation need to be addressed. Carbonaceous materials such as graphene oxide (GO) can overcome such issues by reducing the recombination rate and providing a platform for adsorption accompanied by photocatalytic degradation of TiO2.
1. Conventional Wastewater Treatment Methods for Waste Removal
With the rapid development of industry in recent years, and the manufacturing industry in particular, the amount of untreated organic waste being released into the environment has gradually increased, and it also poses potential health problems to both aquatic life and humans. Many methods have been developed and implemented over the years to control pollution. Conventional wastewater treatment methods can be split into three main branches which are chemical, physical, and biological methods.
Physical methods are methods that separate organic compounds from wastewater via physical means such as adsorption and membrane filtration, which are simple yet effective. Adsorption itself is simple and proved to be effective for dye removal. The raw materials for adsorbent are abundant and the adsorbent can be recycled and regenerated for any future removal if required. However, some adsorbents can be costly themselves [
64]. Examples of common adsorbents used are chitosan [
65] and activated carbon [
66]. Nanofiltration processes are driven by pressure passing through a membrane. The merits of using nanofiltration are that it has a higher flux in comparison to reverse osmosis and a higher rejection rate as compared to ultrafiltration [
67]. Nevertheless, nanofiltration membrane fouling has been a drawback the process brings [
68].
Biological methods introduce the use of microorganisms to degrade and adsorb pollutants in wastewater. Common biological methods utilize plants, also known as phytoremediation and bioremediation by microorganisms. The processes microorganisms used to remove dyes are an aerobic process, an anaerobic process, or a combination of both. Biological methods are still used as they are inexpensive and applicable to many organic wastes [
69]. The problem with this approach is the time required for the organisms to degrade, while some organic compounds such as dyes are resistant to aerobic treatment [
70].
Finally, chemical methods use chemical reagents or chemically created radicals as main components to either oxidize or degrade recalcitrant pollutants. Examples of such methods include coagulation, flocculation, ozonation, ion exchange, and advanced oxidation processes. Coagulation involves adding coagulants into the medium to destabilize the particles, increasing the tendency for them to form agglomerations, followed by flocculation, which allows the formation of larger agglomerates [
71,
72]. For cases of wastewater with high intensity of dye molecules, the process may lead to excessive dependence on coagulant which later forms chemical sludge and is not able to effectively remove dyes with distinct chromophore structures [
73]. Ozone (O
3) has been used to oxidize specific dyes with amine groups and aromatic structures [
74]. However, the oxidation of O
3 has been selective, therefore it is not suitable for some dyes, and as a result, unable to completely degrade organic dyes and can even produce toxic intermediates [
75]. Photocatalysis is one of the promising advanced oxidation process (AOP) methods used to degrade organic wastes into harmless end products of carbon dioxide (CO
2), water molecules (H
2O), and mineral acids.
2. Photocatalysis
Photocatalysis involves a semiconductor in which electrons are excited from the valence band to the conduction band upon irradiation by a light source with energy equivalent or greater to the bandgap of the semiconductor [
76,
77]. The implementation of photocatalysis can overcome the disadvantages of other organic waste removal methods, providing complete degradation of organic pollutants and reducing operation costs because the irradiation source can be from renewable sunlight. As a result, the ease of application has made it one of the commonly practiced methods in wastewater treatment [
78].
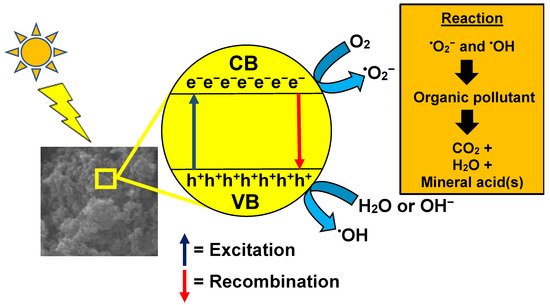
When the photocatalyst is exposed to a light source, the photons will be absorbed by the electrons, causing them to vacate the valence band and travel to the conduction band, leaving a positively charged hole, thus forming an electron-hole pair. The holes at the valence band will oxidize the water molecules nearby to form hydrogen ions and hydroxyl radicals, which are the primary radicals for pollutant degradation [
79]. At the same time, the electron at the conduction band will convert the surrounding oxygen molecules into superoxide radicals. Subsequently, these radicals will tackle the dye molecules and degrade them. The overall process is displayed in
Figure 2. Examples of photocatalysts used in photocatalysis are GO and TiO
2.
Figure 2. Mechanism of photocatalysis for organic waste removal.
2.1. TiO2
TiO2 is an n-type semiconductor with a wide indirect bandgap. The TiO2 structure consists of chains of twisted octahedra of TiO6, where each atom of Ti is surrounded by six oxygen atoms. The tetragonal anatase unit cell contains four units of TiO2 (12 atoms), while the tetragonal rutile unit cell contains two units of TiO2 (6 atoms), and the orthorhombic brookite unit cell contains eight units of TiO2 (24 atoms). Anatase thus has a lower number of cells than rutile and brookite.
Rutile is the most thermodynamically stable phase of the three crystalline phases, which can withstand all temperatures and pressure due to its lower free energy. The anatase and brookite phases are metastable and appear to transition at higher temperatures to the rutile phase. Various sequences, such as anatase to brookite to rutile, brookite to anatase to rutile, brookite to rutile, and anatase to rutile can be used for the phase transformation. These transformations depend on temperature, time, and particle size. Anatase and brookite, at small particle sizes, are very stable. Despite the various advantages of photocatalysts, they have some drawbacks such as that they only can be activated by ultraviolet light, which is only about 5% of the solar spectrum and involves fast recombination of electron-hole pairs, limiting photocatalytic efficiency [
84]. Ways to improve the photocatalytic efficiency of photocatalysts have been studied via doping with metals along with nonmetals, and in combination with other semiconductors [
85,
86].
2.2. GO
In recent years, carbonaceous materials became an interest in studies of photocatalysts due to their physicochemical properties. A well-known allotrope of graphene, GO is primarily a layer of carbon atoms with a combination of sp
2 and sp
3 bonds due to oxidation of graphite, which is a distinct difference from graphene, which possesses only sp
2 hybridized carbon atoms. The sp
3 bonds are mainly attributed to various oxygenated functional groups that can be found covalently bonded on the surface of GO, for example, epoxy, hydroxyl, ketone, and carboxyl groups [
87,
88]. The introduction of functional groups has disrupted the symmetry of the π-network between carbon atoms in the graphene lattice, which restricts GO in many field applications [
89]. However, they allow GO to become hydrophilic and disperse well in water, creating stable dispersions, hence leading to interesting future prospects in nanocomposites [
90,
91]. Functionalized GO-based nanocomposites are common photocatalysts due to their large specific surface areas and remarkable adsorption capabilities, and provide opportunities for further surface modification [
92]. The functional groups that are on the basal plane of graphene provide a large surface area, making GO an excellent adsorbent for the removal of metal ions and dyes from aqueous solutions [
93,
94].
2.3. Production of GO
There are two mainstream approaches to synthesize GO: bottom-up methods whereby simple carbon molecules are used to generate pristine graphene, and top-down methods whereby layers of graphite are used to extract graphene sheets [100]. The drawbacks of bottom-up techniques are that they are time-consuming and are not suitable for large-scale production yet. Bottom-up techniques include chemical vapor deposition, epitaxial growth on silicon carbide, and so on. Therefore, the top-down method would be a more favorable and preferred option in the production of graphene derivatives. The products that are first obtained are graphene oxide or even reduced graphene oxide, which are well established in the application of nanocomposite materials.
2.4. Production of TiO2
2.4.1. Electrophoretic Deposition
Several factors make this the preferred method: efficiency in coating and film fabrication, shorter deposition time, film deposition on non-uniform surfaces, cost effectiveness, tunable thickness of films, homogenous coatings, and simple equipment requirements [
104]. The process is initiated by a DC voltage which activates the charged particles in a suspended solution for deposition onto a substrate. An electric field due to voltage applied to the electrodes interacts with the surface charge of the nanoparticles, leading to the particles migrating to the electrode of opposite charge and the deposition on the electrode, resulting in formation of a homogenous layer.
2.4.2. Spray Pyrolysis
Synthesis techniques such as spray pyrolysis involve a heated substrate, atomizer and a precursor solution (TiCl
3 or Ti
4, etc.) [
105]. This procedure produces thin films by atomizing the solution into tiny droplets, which are then transferred to the heated substrate. Due to the ultrasonic spraying technique used to create the smaller droplets, the atomic cloud aerosol produces larger droplets, at the same time influencing the surface morphology of material produced. Spray pyrolysis is incredibly effective, economical, and requires basic equipment as well. The thin films produced from this method also possess high substrate coverage and potential and homogeneity of mass synthesis. However, the drawbacks of this method include poor quality of thin film, thermal disintegration, and vapor convection, which are factors to be considered for synthesis of TiO
2. Temperature differences cause the vapors to be produced, which prevents the source from adhering to the substrate.
2.4.3. Sol Gel Method
Generally speaking, the sol-gel process entails transforming a system from a liquid “sol” phase, which is mostly in colloidal form, into a solid “gel” phase. Metal organic compounds, such as metal alkoxide and inorganic metal salts, are common precursors in the synthesis of “sol.” A sol or colloidal suspension is created by a succession of hydrolysis and polymerization processes. A wet “gel” will form when a sol is cast into a mold. The gel can be further dried and heated to create solid products.
2.4.4. Sonochemical and Microwave-Assisted Methods
Efficient photoactive TiO
2 nanoparticles can also be synthesized via the sonochemical method while using ultrasonic irradiation for the hydrolysis of titanium tetraisopropoxide (TTIP) in pure water or in an ethanol/water mixture. The concept of acoustic cavitation causes the formation, growth, and collapse of bubbles in the solution while temperature at about 5000 K and pressures at about 1000 atm are the result of cavitational collapse.
2.5. Production of GO-TiO2
2.5.1. Hydrothermal Method
Hydrothermal synthesis indicates a high-temperature and pressure technique for growing crystals from an aqueous solution in an autoclave. The characteristic of water is a solvent with a low boiling point, which allows it to be used under high pressure. Solvents with a high boiling point, such as dimethyl sulfoxide (DMSO), can be expensive and pose potential hazards, thus making water a very attractive option. Fine crystals of the desired nanocomposites are created using increased temperature. Hydrothermal synthesis enables the composition and consistency of the nanocrystals produced to be controlled. The key drawbacks associated with this process, however, are the inability to control material crystal growth (in the autoclave) and the cost of the equipment [
107]. The hydrothermal reaction can also be used to partially reduce GO to graphene.
2.5.2. Solvothermal Method
Solvothermal synthesis, analogous to the hydrothermal method, involves a process for fabricating crystals from non-aqueous organics using an autoclave at high temperatures and pressure [
109]. Compared to the hydrothermal method, the solvothermal method typically has a greater effect on the size, shape, distribution, and crystallinity of the prepared nanocomposites.
2.5.3. Mechanical Mixing
The simplicity and manipulations of the conditions of the reaction have made this strategy gradually gain popularity. This process involves mixing pristine or functionalized TiO2 with GO dispersions, likely accompanied by sonication and stirring to increase the optimum interaction between the precursors of the nanocomposite.
3. Heterojunction of GO/TiO2 Photocatalyst
GO acts as one of the superior support materials for different semiconductors and metals. The photoelectrons generated at the TiO
2 conduction band are rapidly transferred to the graphene layer by the TiO
2 nanoparticle-decorated GO, which promotes the rate of organic dye pollutant degradation. In addition, during photocatalytic reactions, the high surface area of GO provides more surface adsorption sites for contaminants to greatly promote surface photocatalytic reactions, thus improving the catalytic activity. To summarize, graphene oxide has contributed in three ways to improving the photocatalytic degradation of pollutants: (1) improvement of the surface area of TiO
2 due to its interaction with the two-dimensional matt structure of GO; (2) improvement of the adsorption of aromatic pollutants due to their strong π-π interactions with the aromatic network of GO; and (3) reduction of the recombination rate between the positive holes and the photogenerated electrons due to the significant electronic conductivity of GO, which functions as an electron sink for photogenerated electrons on the TiO
2 surface [
110,
111].
4. Advantages of GO/TiO2 Composite Structure
Research carried out by [113] showed that the surface area of GO/TiO2 composite (78.12 m2/g) is larger than that of bare TiO2 (57.01 m2/g) through the nitrogen adsorption/desorption measurement using the Brunauer–Emmett–Teller (BET) equation. The researchers showed that increase in the surface area enhanced the pollutant adsorption ability of GO/TiO2 composite to approximately 37% compared to that of the produced bare TiO2. The contact area between the produced photocatalyst and pollutants is enhanced [114]. Thus, the photodegradation performance of GO/TiO2 composite is better than that of bare TiO2. A large surface area provides more active sites for redox reaction [115] including separation and transfer of photogenerated electron-hole pairs [116], thus enhancing the photodegradation performance. Besides, large surface area also improves the utilization of light [116].
Band gap absorption edge of bare TiO2 is 440 nm, which mostly adsorbs UV light to initiate the photodegradation process. GO has great absorption in the visible light range [114]. Thus, when TiO2 combines with GO, the absorption range of GO/TiO2 composite can be expanded up to a wavelength range of 800 nm [113]. Compared with GO and TiO2, the light absorption characteristics of GO/TiO2 composite are enhanced. The generated Ti3+ enhances the visible light absorption range [114]. Research by [113] showed that prepared bare TiO2 had a bandgap reading of 3.20 eV and the bandgap reading of GO/TiO2 composite was 2.80 eV using a Tauc plot. This outcome indicates GO/TiO2 composite can absorb visible light effectively and therefore, enhance the photodegradation performance under visible light irradiation.
GO/TiO2 composite possess large surface area and a low photogenerated electron-hole pairs recombination rate to degrade the pollutant. Other than that, the visible light absorption range of the GO/TiO2 composite can also be enhanced due to the response of graphene to visible light [114]. Electrochemical impedance spectroscopy (EIS) and photocurrent study can be carried out using a three-electrode system consisting of FTO covered with a sample as a working electrode, a platinum plate as counter electrode, and Ag/AgCl as a reference electrode.
5. GO-TiO2-Related Nanocomposites in Wastewater Treatment and Their Findings
The GO-TiO2 heterostructure is one of the most significant and frequently studied semiconductor photocatalysts for the decomposition of organic pollutants [119]. Factors such as high surface area, excellent conductivity, and chemical stability allow carbonaceous materials such as graphene oxide to be a compactible surface modification combination with titanium dioxide. Hence, a plethora of studies have investigated and reported on graphene oxide-coupled TiO2, including other semiconductor materials for enhanced photocatalytic performance over organic pollutant degradation. Lin et al. reported on synthesized reduced graphene oxide decorated with titanium dioxide nanocomposites via a combination of ultrasonication and hydrothermal reaction which involved titanium tetrachloride (TiCl3) and graphene oxide (GO) as precursors [120]. As a result of combining the two components, the adsorption and degradation of methyl orange (MO) were significantly increased. The prepared sample was able to remove up to almost 90% of the dye, which was nearly threefold the rates of bare TiO2 and GO under ultraviolet lamp irradiation for 4 h. The process having lower degradation efficiency in sunlight than using ultraviolet illumination is due to the excitation of electrons in the conduction band being made easier by the high energy of ultraviolet light. The merit of a hybrid allows a greater surface area which contributes to the adsorption of MO onto GO surfaces via π-π interaction, followed by degradation of MO by active groups generated by TiO2, feats that cannot be accomplished by bare TiO2 and GO alone.
6. GO/TiO2 Photocatalytic Mechanism
The application of GO/TiO
2 for the removal of aromatic pollutants such as dyes (methylene blue and methyl orange) reflects the efficiency of GO/TiO
2 nanocomposites. Equations (1)–(8) describe the route taken by the photogenerated electrons followed by the decomposition of aromatic pollutants by GO/TiO
2. The proposed mechanism of formation of radicals for the photodegradation of pollutants is in agreement with [
126]. Lin et al. [
120] also stated that dye molecules were initially adsorbed onto the surface of GO/TiO
2 under the influence of π-π bonds of a graphene sheet.
The irradiation source then initiates the photogeneration of electron-hole pairs on the surface of photocatalyst. The resultant hole will break apart water molecules into hydrogen ions and hydroxyl radicals (OH•). The resultant electrons in the valence band of TiO2 are transferred to the conduction band of GO to convert oxygen molecules into hydrogen peroxide and subsequently OH•. This whole process repeats as long as the irradiation source still remains active.
Band edge positions of the photocatalysts forming the GO/TiO
2 composite have been determined for better estimation of the photogenerated charge carrier separation mechanism. The conduction band (CB) and valance band (VB) potentials of the photocatalysts represented by
ECB and
EVB can be calculated using Equations (9) and (10) [
144] as below:
where Eg represents bandgap energy which can be obtained through the Tauc plot, X represents absolute electronegativity, and Ee represents energy of free electrons on the hydrogen scale which has an approximate reading of 4.50 eV.
On the other hand, Mott-Schottky (MS) can be utilized to determine the rationality of the
ECB and
EVB readings calculated through the previous equations. By using the MS plot, we are able to determine the type of semiconductor or metal oxide. Based on the literature, GO is a p-type semiconductor whereas TiO
2 is an n-type semiconductor [
145].
The flat band potential can be converted to normal hydrogen scale using Equation (11) [
145] below:
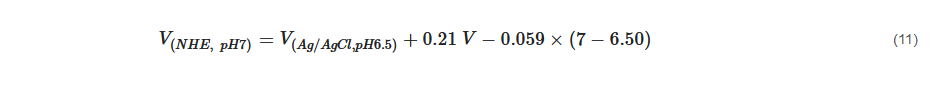
7. Factors Affecting the Degradation of Organic Pollutants
Due to variations in the lattice structure, morphology, surface area, particle size, as well as impurities on the catalyst surface, different photocatalysts will have different photocatalytic activities, thus, influencing the adsorption of pollutants at the surface of the photocatalyst, and the rate of recombination of electron-hole pairs. One of the important variables that will affect the performance of dye degradation is pH as it influences the dye reactions in several ways. Adsorption of pollutants onto the surface of the photocatalyst can contribute to the efficiency of the photocatalyst in the removal of pollutants. Pollutant adsorption depends on the initial pollutant concentration in the solution. The initial concentrations of the pollutant can thus influence the process of photodegradation. The pollutants that are adsorbed on the surface and not the bulk of the solution are involved in the process. High concentrations of pollutants will increase the turbidity of water and cover more active sites. Consequently, fewer photons will reach the surface; fewer OH2 species will therefore be produced, leading to a decrease in the efficiency of degradation.
The optimum amount of photocatalyst needed usually depends on the concentration of the pollutant and the volume of the solution in which the pollutants are mixed. As the concentration of photocatalyst is below the optimum level, the rate of photocatalysis will gradually increase along with the increase in photocatalyst loading. This can be explained due to the increase in available active sites on the photocatalyst surface, and the number of radicals generated from the photocatalyst to degrade and mineralize the pollutant. However, when the photocatalyst is further added beyond the optimum level, the rate of photodegradation gradually decreases. At high concentrations, the photocatalyst will likely agglomerate and thus lead to unfavorable scattering of light and fewer photons can reach the surface of the photocatalyst [151].
This entry is adapted from the peer-reviewed paper 10.3390/nano12193536