Iron oxide nanoparticles are frequently used in various biomedical applications, in particular as magnetic resonance imaging contrast agents in liver imaging. Indeed, number of iron oxide nanoparticles have been withdrawn due to their poor clinical performance and/or toxicity issues. In the literature it has been criticized that failure of clinical applications is to a large extent due to poor understanding of the sub-cellular molecular targets of nanoparticles. Nanoparticles were found to change the activity of autophagic flux via lysosome-dependent signalling. However, precise underlying mechanisms of such modulation remain poorly understood.
- nano–bio interactions
- iron oxide nanoparticles
- autophagy
- lysosomes
- magnetic resonance imaging
1. Introduction
Iron oxide nanoparticles (NPs) showed to be very useful in different biomedical applications [1-3]. Range of iron oxide NPs applications in biology and medicine is enormous, including targeted drug delivery, imaging, biosensors, and different therapeutic modalities [1][2][3][4][5][6][7]. Contrast agents for magnetic resonance imaging were the first nanoparticles to be approved for clinical use [8][9]. However, very soon those contrast agents have been withdrawn or discontinued due to various side effects [8][9][10][11], clearly indicating their overlooked cytotoxic potential.
Last year the U.S. National Cancer Institute (NCI) in Bethesda, Maryland cancelled funding for nanotech research centres [12]. This resulted in a swirl in bionano research, flooding the scientific literature with debates concerning clinical efficacy of nanotechnologies [13][14][15][16][17]. Recent studies of world-leading scientists in the area of nanoresearch pushed it towards a better understanding of fundamental intracellular signal modulations induced by nanomaterials [18][19]. Under a burden of accumulating evidence researchers start to realize that our current approach in bionano field is broken, and that is why we have not observed significant clinical translation of nanomedicines.
2. Data
Studies on nanotoxicity showed that iron oxide NPs induce oxidative stress due to excessive reactive oxygen species (ROS) accumulation [20][21][22][23][24]. Additionally, it was found that NP may induce significant cellular responses without noticeable oxidative stress [25]. Indeed, subcytotoxic doses of NPs trigger significant alteration in subcellular signalling [26]. However, more thorough studies are definitely needed in order to decipher sub-cellular molecular targets of NPs.
In fact, researchers start realizing that the liver is a predominant end point of the majority of intravenously administered nanoparticles [27]. Particles with diameter of ~ 150–200 nm have been shown to penetrate the space of Disse and interact with hepatocytes [28]. As a matter of fact, there are little known about molecular targets of interactions of hepatic cells with iron oxide NPs [27]. A direct comparison of the observed effects on closely related cell lines is lacking [27][28].
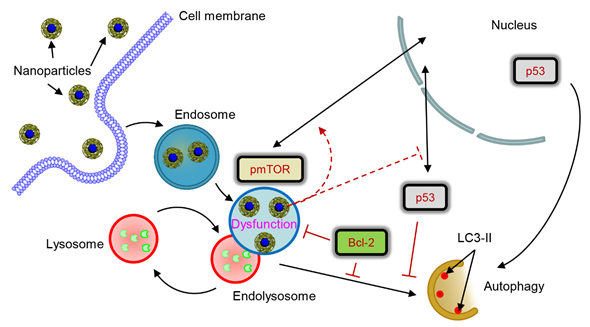
In the study we investigated how lysosomal-mediated signalling is changed upon iron oxide nanoparticle treatment in hepatic cells [29]. Our data showed that sub-lethal treatment with iron oxide NP leads to NP accumulation in lysosomes. Such NP accumulation in lysosomes results in drastic changes of lysosomal size and shape and, as a result, progressive impairment of lysosomal function. Lysosomal impairment in turn alters sub-cellular localization of pmTOR and p53 proteins. Further changes of sub-cellular localization of p53 protein induced by nanoparticle result in deregulation of the autophagic flux. Importantly, high levels of Bcl-2, that are expressed by certain cell lines, counteract autophagy initiated by nanoparticles. Altogether our data identify lysosomes as a central hub that controls nanoparticle-mediated responses in hepatic cells.
Figure 1. Scheme of lysosomal dysfunction upon nanoparticle treatment. Sub-lethal doses of iron oxide nanoparticles are endocytosed into lysosomes in hepatic cells within 12 h. Accumulation of nanoparticles in lysosomal compartments leads to progressive impairment of lysosomal function. The resulted lysosomal dysfunction probably affects sub-cellular localization of pmTOR and p53. Progressive lysosomal dysfunction leads to initiation of autophagic flux, which is supported by nuclear p53. Contrary cytosolic p53 and high levels of Bcl-2 inhibit autophagy. nanoparticle-induced autophagic flux is regulated by interplay between p53-mTOR axis and Bcl-2 signaling in hepatic cells.
This entry is adapted from the peer-reviewed paper 10.3390/cells9041015
References
- Wu, W.; Jiang, C.Z.; Roy, V.A. Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale 2016, 8, 19421-19474.
- Shi, J.J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: progress, challenges and opportunities. Nature Reviews Cancer 2017, 17, 20-37.
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Reviews Drug Discovery 2008, 7, 771-782.
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284-3307.
- Martinez-Banderas, A.I.; Aires, A.; Quintanilla, M.; Holguin-Lerma, J.A.; Lozano-Pedraza, C.; Teran, F.J.; Moreno, J.A.; Perez, J.E.; Ooi, B.S.; Ravasi, T., et al. Iron-based core-shell nanowires for combinatorial drug delivery and photothermal and magnetic therapy. ACS Appl Mater Interfaces 2019, 11, 43976-43988.
- Lunov, O.; Uzhytchak, M.; Smolkova, B.; Lunova, M.; Jirsa, M.; Dempsey, N.M.; Dias, A.L.; Bonfim, M.; Hof, M.; Jurkiewicz, P., et al. Remote actuation of apoptosis in liver cancer cells via magneto-mechanical modulation of iron oxide nanoparticles. Cancers (Basel) 2019, 11, 1873.
- Uzhytchak, M.; Lynnyk, A.; Zablotskii, V.; Dempsey, N.M.; Dias, A.L.; Bonfim, M.; Lunova, M.; Jirsa, M.; Kubinova, S.; Lunov, O., et al. The use of pulsed magnetic fields to increase the uptake of iron oxide nanoparticles by living cells. Applied Physics Letters 2017, 111, 243703.
- Wang, Y.-X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quantitative Imaging in Medicine and Surgery 2011, 1, 35-40.
- Wang, Y.X.J.; Idee, J.M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quantitative Imaging in Medicine and Surgery 2017, 7, 88-122.
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm Res 2016, 33, 2373-2387.
- Kendall, M.; Lynch, I. Long-term monitoring for nanomedicine implants and drugs. Nat Nanotechnol 2016, 11, 206-210.
- https://www.sciencemag.org/news/2019/05/us-cancer-institute-cancels-nanotech-research-centers.
- Park, K. The beginning of the end of the nanomedicine hype. J Control Release 2019, 305, 221-222.
- Lammers, T.; Ferrari, M. The success of nanomedicine. Nano Today 2020, 31, 100853.
- McNeil, S.E. Evaluation of nanomedicines: stick to the basics. Nature Reviews Materials 2016, 1, 16073.
- Leong, H.S.; Butler, K.S.; Brinker, C.J.; Azzawi, M.; Conlan, S.; Dufes, C.; Owen, A.; Rannard, S.; Scott, C.; Chen, C., et al. On the issue of transparency and reproducibility in nanomedicine. Nat Nanotechnol 2019, 14, 629-635.
- Wilhelm, S.; Tavares, A.J.; Chan, W.C.W. Reply to "Evaluation of nanomedicines: stick to the basics''. Nature Reviews Materials 2016, 1, 16074.
- Cheng, Y.H.; He, C.L.; Riviere, J.E.; Monteiro-Riviere, N.A.; Lin, Z.M. Meta-analysis of nanoparticle delivery to tumors using a physiologically based pharmacokinetic modeling and simulation approach. Acs Nano 2020, 14, 3075-3095.
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials 2016, 1, 16014.
- Hsiao, J.K.; Chu, H.H.; Wang, Y.H.; Lai, C.W.; Chou, P.T.; Hsieh, S.T.; Wang, J.L.; Liu, H.M. Macrophage physiological function after superparamagnetic iron oxide labeling. NMR Biomed 2008, 21, 820-829.
- Lunov, O.; Syrovets, T.; Buchele, B.; Jiang, X.; Rocker, C.; Tron, K.; Nienhaus, G.U.; Walther, P.; Mailander, V.; Landfester, K., et al. The effect of carboxydextran-coated superparamagnetic iron oxide nanoparticles on c-Jun N-terminal kinase-mediated apoptosis in human macrophages. Biomaterials 2010, 31, 5063-5071.
- Lunov, O.; Syrovets, T.; Rocker, C.; Tron, K.; Nienhaus, G.U.; Rasche, V.; Mailander, V.; Landfester, K.; Simmet, T. Lysosomal degradation of the carboxydextran shell of coated superparamagnetic iron oxide nanoparticles and the fate of professional phagocytes. Biomaterials 2010, 31, 9015-9022.
- Bae, J.E.; Huh, M.I.; Ryu, B.K.; Do, J.Y.; Jin, S.U.; Moon, M.J.; Jung, J.C.; Chang, Y.; Kim, E.; Chi, S.G., et al. The effect of static magnetic fields on the aggregation and cytotoxicity of magnetic nanoparticles. Biomaterials 2011, 32, 9401-9414.
- Mirshafiee, V.; Sun, B.; Chang, C.H.; Liao, Y.P.; Jiang, W.; Jiang, J.; Liu, X.; Wang, X.; Xia, T.; Nel, A.E. Toxicological profiling of metal oxide nanoparticles in liver context reveals pyroptosis in kupffer cells and macrophages versus apoptosis in hepatocytes. ACS Nano 2018, 12, 3836-3852.
- Ma, X.; Hartmann, R.; Jimenez de Aberasturi, D.; Yang, F.; Soenen, S.J.H.; Manshian, B.B.; Franz, J.; Valdeperez, D.; Pelaz, B.; Feliu, N., et al. Colloidal gold nanoparticles induce changes in cellular and subcellular morphology. ACS Nano 2017, 11, 7807-7820.
- Lunova, M.; Smolkova, B.; Lynnyk, A.; Uzhytchak, M.; Jirsa, M.; Kubinova, S.; Dejneka, A.; Lunov, O. Targeting the mTOR signaling pathway utilizing nanoparticles: A critical overview. Cancers (Basel) 2019, 11, 82.
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J Control Release 2016, 240, 332-348.
- Tsoi, K.M.; MacParland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M., et al. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater 2016, 15, 1212-1221.
- Uzhytchak, M.; Smolkova, B.; Lunova, M.; Jirsa, M.; Frtus, A.; Kubinova, S.; Dejneka, A.; Lunov, O. Iron oxide nanoparticle-induced autophagic flux is regulated by interplay between p53-mTOR axis and Bcl-2 signaling in hepatic cells. Cells 2020, 9, 1015.