Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Integrative & Complementary Medicine
Multisystem inflammatory syndrome in children (MIS-C) has been widely reported in some children diagnosed with SARS-CoV-2. Clinical signs of MIS-C are manifested at 2 to 4 weeks after SARS-CoV-2 infection, where elevated biomarkers of inflammation and cardiac dysfunction are the hallmark of this syndrome when infection or exposure to SARS-CoV-2 has been confirmed.
- multisystem inflammatory syndrome
- children
- COVID-19
1. MIS-C Case Definition and Clinical Manifestations
In April 2020, the Paediatric Intensive Care Society recognized a critically ill in children with characteristics of hyperinflammatory shock and evidence of SARS-CoV-2 infection. The RCPCH introduced the term PIMS-TS and subsequently, the CDC and WHO published case definitions for MIS-C in May 2020 [15,16].
According to WHO, children and adolescents (0–19 years) showing signs of MIS-C have previous persistent fever for more than three days with any two of the following conditions: external signs of inflammation (rash or bilateral non-purulent conjunctivitis and oral cavity, hand, or foot alterations), hypotension or shock, cardiac abnormalities, signs of coagulopathy, or acute gastrointestinal conditions [17]. CDC considers MIS-C for individuals aged <21 years presenting fever for at least 24 h, laboratory evidence of inflammation, evidence of clinically severe illness requiring hospitalization, and organ involvement (respiratory, cardiac, renal, hematologic, gastrointestinal, dermatologic, or neurological) [18]. These clinical symptoms require accompaniment by laboratory findings of inflammation such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) or procalcitonin (PCT) levels [19], and evidence of SARS-CoV-2 infection or contact with COVID-19 patients ruling out bacterial sepsis, staphylococcal or streptococcal shock syndromes caused by infection with other pathogens [3]. Only CDC considers hospitalization time as a criterion for MIS-C definition, and RCPCH did not include SARS-CoV-2 positivity or epidemiologic link (Figure 1) [16].
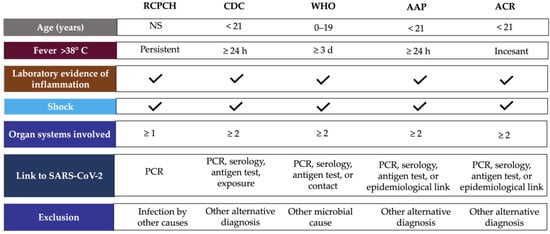
Figure 1. MIS-C case definition according to international guidelines. Abbreviations: AAP, American Academy of Pediatrics; ACR, American College of Rheumatology; CDC, Centers for Disease Control and Prevention; NS, no specified; PCR, polymerase chain reaction, RCPCH, Royal College of Paediatrics and Child Health; WHO, World Health Organization.
The American Academic of Pediatrics (AAP) defined MIS-C as the syndrome of an individual under 21 years of age presenting fever, laboratory evidence of inflammation, and proof of clinically severe disease requiring hospitalization, with multisystem (≥2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neuro-logic), without a plausible alternative diagnosis, and positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen testing; or exposure to COVID-19 within the four weeks before the onset of symptoms [20]. In addition, the American College of Rheumatology (ACR) published clinical guidance to define a case of MIS-C, which includes: incessant fever (greater than 38 °C), epidemiological link to SARS-CoV-2, and at least two suggestive clinical features (rash, changes in oral mucosa, conjunctivitis, neurological symptoms, edema of hands/feet) (Figure 1) [21].
Clinical signs of MIS-C appear 2–4 weeks after SARS-CoV-2 infection, with a significant proportion (75%) of antibodies to class-switched viral antigens indicating that most, if not all, cases of MIS-C are the result of previous or unclear SARS-CoV-2 infection [5,22,23]. However, the range of SARS-CoV-2 virus detected by real-time polymerase chain reaction (RT-PCR) is widely spread among children with MIS-C. It varies from 21% to 40% in studies involving either method for the detection of SARS-CoV-2 [24,25,26,27].
The diagnosis method for SARS-CoV-2 infection is also controversial. RT-PCR and antigen detection are relative indicators of viral load. SARS-CoV-2 spike (S) antigens were detectable in the blood of children with MIS-C [28]. However, N and S antigens in acute COVID-19 did not correlate strongly with RT-PCR [29]. On the other hand, the use of a novel method (MSD S-PLEX CoV-2 N and S assays) demonstrated that, during the early hospital course, SARS-CoV-2 N and S antigens are detectable in blood in most pediatric patients with acute COVID-19, but in few cases of MIS-C [30]. Therefore, the RT-PCR method for COVID-19 detection is not exclusive to MIS-C diagnosis, and serology and epidemiological linkage are also considered. Currently, the ACR emphasizes that MIS-C diagnosis should be confirmed on the basis of the totality of history, physical examination, and laboratory studies [21].
Like KD, patients with MIS-C have different features of cardiac dysfunction, such as valvulitis, coronary artery dilatation, myocardial dysfunction, and myocarditis [31,32,33]. In severe cases of MIS-C, patients require cardiac or respiratory support [34,35]. Therefore, cardiac biomarkers and echocardiography should be monitored during the hospital stay. The ACR has recommended monitoring troponin T and B-type natriuretic peptide (BNP)/N-terminal proBNP (NT-proBNP) and assessment of BNP/NT-proBNP levels to distinguish between MIS-C patients with and without left ventricular (LV) dysfunction [21]. However, a meta-analysis of laboratory cardiac markers for children with MIS-C and COVID-19 revealed that only BNP was the key cardiac marker that showed differences between patients with non-severe MIS-C and severe COVID-19 and between non-severe and severe MIS-C patients. Meanwhile, neither troponin nor aspartate aminotransferase showed notable differences in cardiac injury between MIS-C and COVID-19 patients [36]. Nevertheless, coronary artery aneurysms regressed in the first month in 80% of patients with MIS-C, and this was not observed in KD patients [21,24,37].
Furthermore, MIS-C differs from KD concerning the age at presentation, as MIS-C typically affects the oldest children and adolescents (with a range of 6 to 12 years), unlike KD, which is more common before the age of 5 years [3,27,37,38]. Other interesting findings include that severe manifestations of MIS-C occur less frequently in Caucasians compared to the frequency expected in the general population (many of whom are of African-American or Afro-Caribbean ethnicity) [23,38,39]. In addition, the ACR panel considers that patients with MIS-C more commonly manifested LV dysfunction, shock, gastrointestinal, and neurological symptoms than patients with KD [21]
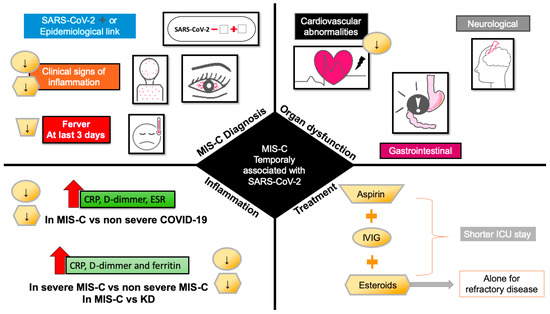
2. Inflammatory Markers in MIS-C
Even though the immunopathologic mechanisms of MIS-C remain poorly understood, high inflammatory markers have been identified, and patients with MIS-C were found to respond appropriately to therapy with immunomodulators or anti-inflammatory drugs [40,41,42,43]. Due to the clinical course of MIS-C and its high variability, identification of the distinct cellular, chemokines, cytokines, coagulation, and inflammatory markers is essential to comprehend clinical evolution. In addition, it has been suggested that cells involved in the innate and adaptive immune response are affected, as well as important markers of coagulation and cardiac and hepatic function [44].
Most children with MIS-C presented anti-SARS-CoV-2 IgG antibodies, indicating a past infection of at least 2–3 weeks (Table 1) [40]. The study by Anderson et al. [45] also suggests that children with MIS-C have high SARS-CoV-2 spike immunoglobulin G (IgG) titers compared with children with severe COVID-19. In addition, autoantibodies directed against endothelial, gastrointestinal, and immune cells were found [46].
The first class of clinical parameters reported associated hyperinflammation, including elevated acute phase reactants [5,41,44,47], accompanied by increased biomarkers of coagulation [41,47,48,49] and cardiac function [39,43,50,51]. In the acute phase of MIS-C, exacerbation of cytokines as some interleukins (IL), tumor necrosis factor-alpha (TNF-a), and interferon-gamma (INF-γ) levels have been reported [48,51,52,53,54,55], as well as chemokines including the IL-2 receptor agonist, C-C motif chemokine ligand 2 (CCL2), C-X-C motif chemokine ligands 8, 9 and 10 (CXCL8, CXCL9, CXCL10), and monocyte chemoattractant protein (MCP)-1 [42,48,56,57,58]. In addition, changes in leukocyte count and distribution are considered as circulating biomarkers [54,59,60,61,62], as well as significant changes in serum biomarkers such as albumin [26,44,63,64], lactate dehydrogenase (LDH) [41], creatinine [41,48], sodium [48,57,63], triglycerides [65,66] and zonulin [67,68] (Table 1).
Table 1. MIS-C circulating biomarkers altered.
| Category | Biomarkers | References |
|---|---|---|
| Antibodies | Anti-spike IgG e IgA | [46] |
| Acute phase reactants | ↑ C-reactive protein, procalcitonin, ferritin, erythrocyte sedimentation rate | [5,41,44,47] |
| Coagulation | ↑ D-dimer, fibrinogen, prothrombin T, partial thromboplastin time | [41,47,48,49] |
| Cardiac function | ↑ Troponin, brain type natriuretic peptide (BNP), Pro-BNP | [39,43,50,51] |
| Cytokines | ↑ IL-1a, IL-2, IL-6, IL-8, IL-17, IL-33, TNF-a, IFNγ | [48,51,52,53,54,55] |
| Chemokines | ↑ CCL2, CXCL8, CXCL9, CXCL10, MCP-1 | [42,48,56,57,58] |
| Monocytes | ↓ Monocyte HLA-DR and CD86+ | [52,69] |
| Dendritic cells | ↓ Plasmacytoid dendritic cells | [56,69] |
| Platelets | ↓ Total count of platelets | [24,50,53,70] |
| Neutrophils | ↑ Total count of neutrophils | [24,59,60,61,62,71] |
| Natural killer | ↓ CD16+, CD56+ ↑ CD38+ |
[60,69,72] |
| Lymphocytes B | ↑ Plasmablasts, naive B cells | [59,60,73] |
| Lymphocytes T | ↓ CD4+, CD8+ | [52,62,73,74,75] |
| Other laboratory markers | ↓ Albumin, sodium ↑ Lactate dehydrogenase, alanine transaminase, creatinine, triglycerides, creatine kinase, blood urea nitrogen, zonulin |
[26,41,44,45,48,63,64,65,66,67] |
Upward arrows indicate increased biomarker levels. Down arrows indicate decreased biomarker levels.
MIS-C and severe COVID-19 have prominent systemic inflammation [68]. However, recent evidence suggests some differences in the inflammatory profile in MIS-C versus COVID-19, as analyzed by Zhao et al. [54], where children with MIS-C had lower levels of LDH, total platelet count (PLT) and higher levels of ESR compared to children with severe COVID-19. In contrast, lower levels of absolute lymphocyte count (ALC) and higher levels of CRP, D-dimer, and absolute neutrophil count (ANC) were observed in patients with MIS-C compared to non-severe COVID-19. In addition, patients with severe MIS-C had increased levels of leukocytes, CRP, D-dimer, and ferritin compared to non-severe MIS-C. In addition, MIS-C showed higher levels of CRP, D-dimer, ferritin, and creatinine and low levels of leukocytes, ALC, PLT, albumin, and sodium versus KD [64]. Therefore, the evolution of inflammatory markers could be useful in order to assess the severity of MIS-C [72].
3. MIS-C Treatment
MIS-C treatment focuses on the clinical stabilization of hospitalized patients and the prevention of multi-organic damage and long-term sequelae; for non-hospitalized patients, antiplatelet agents have shown promising results. The ARC recommends using aspirin for 3–5 mg/kg/day in patients without bleeding [21].
MIS-C treatment in hospitalized patients is based on the KD approach, focused on the use of IVIG and glucocorticoids [83,84,85,86,87]. The AAP, ACR, American Heart Association (AHA), Helen DeVos Children’s Hospital Foundation (HDVCH), and Infection Diseases Society of America (IDSA), as well as other worldwide health organizations, recommend the continued use of IVIG at a dose of 1–2 g/kg, steroid therapy (2–3 mg/kg/d), and antiplatelet therapy (aspirin) [88].
The use of IVIG has been recommended in KD patients to reduce coronary artery abnormalities [89,90], while its benefit in myocarditis remains unclear because the successful use of IVIG in coronavirus-associated myocarditis has been supported only by case reports [21]. The American Heart Association (AHA) suggests that, although the mechanism of action of IVIG is unknown, there is a modulation of cytokine production, neutralization of toxins, augmentation of regulatory T-cell activity, and regulation of antibody synthesis [91,92].
Using glucocorticoids in combination with IVIG is more effective than monotherapy with IVIG in patients without contraindications to glucocorticoids and is associated with shorter ICU stays [50,93]. The monotherapy with glucocorticoids needs more investigation, and the experts do not recommend its use alone until more evidence is feasible (Figure 2).

Figure 2. Effect of pharmacological treatment for MIS-C on clinical signs at admission, organ dysfunction and inflammation. Downward arrows indicate the decrease in signs, symptoms, and markers of inflammation by treatment with aspirin (trapezoid), IVIG (circle), and steroids (hexagon). Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ICU, intensive care unit; IVIG, intravenous immunoglobulin.
The use of antibiotic therapy is only recommended by AAP for mild and severe illness or shock. Anticoagulant therapy is suggested as prophylaxis or therapy in patients with eject fraction <35% or thrombosis evidence [88]. In patients with a higher risk of complications by IVIG or refractory disease, the experts recommend the intensification with higher doses of glucocorticoids, as well as the use of anakinra, a recombinant human IL-1 receptor antagonist [91,92,94,95], or the TNF-a inhibitor infliximab [21,93,96].
Given that hyperinflammation in MIS-C is associated with SARS-CoV-2 infection, it is important to know non-pharmacological interventions in clinical trials to reduce inflammation during COVID-19 or improve patient prognosis during hospitalization. In the search for nutraceutical compounds as adjuvants in KD, TSS, or COVID-19 in children, nutraceutical interventions have only been carried out in adult subjects with COVID-19. We found only one intervention with vitamin C in children with KD to evaluate changes in the diameter of the brachial artery [121]. In clinical trials, the most commonly used nutraceuticals with anti-inflammatory and antioxidant properties tested as complementary treatment versus COVID-19 were curcumin, omega-3 fatty acids, quercetin, and vitamins A, C, and D3.
After more than two years of the COVID-19 pandemic, the pathomechanisms of MIS-C are not yet fully understood, and it is well-recognized that cytokine storm is a key to organ dysfunction. Therefore, the use of IVIG and steroids are the pharmacological recommended treatments. However, complementary therapy based on natural compounds could be feasible by its potential antioxidant and anti-inflammatory activities. As many combinations could have these actions, the perspective of the use of nutraceuticals used for COVID-19 in adults as curcumin, omega-3 fatty acids, and vitamins (A, C, D, and E), have been shown promised results by their ability to reduce inflammatory markers and better prognostic during the hospital stay.
This entry is adapted from the peer-reviewed paper 10.3390/life12101652
This entry is offline, you can click here to edit this entry!
