1. Pathogen Detection
The presence of pathogenic microorganisms in agri-food systems is a growing global concern for human and animal health. If not promptly detected, the spread of these pathogens through the food supply chain can become a major problem causing illnesses and loss of lives. Hence, simple and rapid biosensors are being developed for the detection of pathogens.
Wang et al. [
82] developed an electrochemical and colorimetric signal-producing biosensor for the on-site detection of
Vibrio parahaemolyticus (VP). VP is a gram-negative bacteria found in the ocean and estuaries that can cause inflammatory gastroenteritis in humans [
112]. The VP detection system was developed using phenylboronic acid and ferrocene-modified platinum (Pt)-doped Ti
3C
2 MXene nanocomposites (PBA-Fc@Pt@MXene). The use of PBA-Fc@Pt@MXene provided high electrical conductivity and significantly enhanced the electrochemical signal intensity and produced a color change when the existence of hydrogen peroxide (H
2O
2) and 3,3′,5,5′-tetramethylbenzidine (TMB) was detected. Incorporated with a VP aptamer, this biosensor can both voltammetrically determine and colorimetrically visualize the presence of VP as low as 5 CFU/mL and 30 CFU/mL, respectively. The linear range for electrochemical measurement and colorimetric determination is 10–10
8 CFU/mL and 10
2–10
8 CFU/mL, respectively. This fabricated biosensing device provides a dual-mode detection method for highly effective VP detection and measurement.
Salmonella typhimurium (ST) is another dangerous foodborne pathogen that causes high morbidity and mortality [
113]. A novel dual-target fluorescent aptasensor was fabricated for live ST and VP analysis [
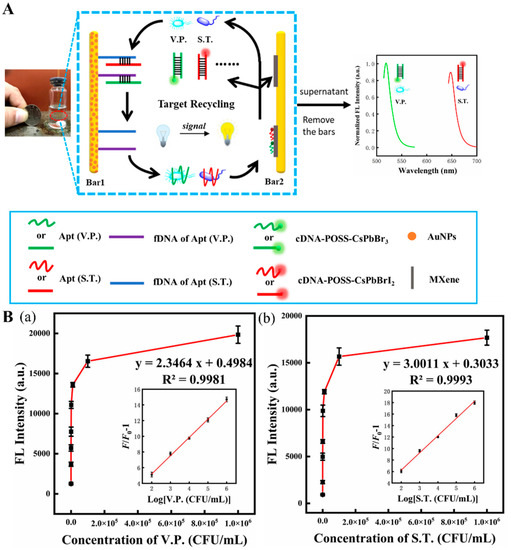
81]. As shown in
Figure 6A, this fluorescent device was assembled by two magnetic bars (1 and 2), where bar 1 was used as a biorecognizer for target recognition and capture, and bar 2 served as a signal amplifier that released the enhanced fluorescent signal. Bar 1 was coated with aptamers capable of recognizing and capturing two bacteria strains. Bar 2, modified with Ti
3C
2 MXene nanosheets, served as a platform to support and immobilize complementary DNA (cDNA) tailed with polyhedral oligomeric silsesquioxane-perovskite quantum dots probes (cDNA-POSS-PQDs). When the two target bacteria were present, they were captured by their specific aptamers on bar 1 and subsequently released with their aptamers into the aqueous supernatant. Due to the agitation, bacteria–aptamer conjugate attached to the cDNA on bar 2 and then tore away the cDNA-POSS-PQDs from bar 2, which finally provided a fluorescent response in the solution. The live bacteria can be released from the aptamer and become free again due to the reaction between the aptamer and cDNA-POSS-PQD with low Gibbs free energy (∆G < 0), leading them to trigger the next detection cycle. Ultimately, the fluorescent response was amplified and reached a maximum that significantly increased the biosensor sensitivity. LODs of 30 CFU/mL and 10 CFU/mL, respectively, for ST and VP, and a linear range of 10
2 to 10
6 CFU/mL were obtained (
Figure 6(Ba,Bb)). Therefore, this biosensor can sensitively detect the target bacteria and differentiate the live from the dead ones.
Figure 6. (
A) Scheme of fluorescent aptasensor for dual-targets detection with its signal amplification strategy. (
B) (
a,
b) the fluorescence intensity of the aptasensor in the presence of VP (
a) and ST (
b). Inset: linear range for this aptasensor for two corresponding foodborne pathogens. Reproduced with permission from Ref. [
81].
Simply using MXene and MXene-based nanocomposites for the NA immobilization yields only weak, unstable signals due to the activity of the live pathogens which may (1) escape or detach from the aptamer capture and stay free in the solution, and (2) disturb the signal when they are struggling with the aptamer capture. Hence, a MXene-based signal amplifier is used in NA biosensors to facilitate the on-site determination of live pathogens.
2. Mycotoxins Detection
Mycotoxins are toxic secondary fungal metabolites found in plant and animal foods. The consumption of some mycotoxin-containing foods beyond certain recommended amounts can cause serious health issues, including death. A sensor for the detection of mycotoxins in food and feed products will help enable a strategy to mitigate adverse health effects in humans and animals.
Zhang et al. [
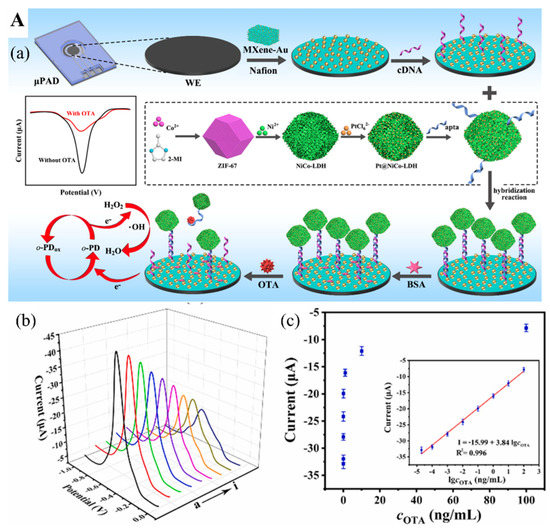
87] developed a switchable signal electrochemical aptasensor for the detection of mycotoxin Ochratoxin A (OTA) via a substrate-aptamer-signal amplifier sandwich structure. The OTA aptamer cDNA with a thiol group (-SH) was immobilized on the surface of MXene/Au nanocomposites-coated paper device via Au-S covalent bond (
Figure 7(Aa)), employed as the transducer substrate coated on the working electrode. OTA aptamers tailed with peroxidase mimic nanostructure based on Pt nanoparticles-doped NiCo hollow layer double hydroxides (Pt@NiCo-LDH) were adopted to hybridize with their cDNA to form a rigid dsDNA helix, which kept the “signal-on” state (
Figure 7(Aa)). As the OTA-contaminated sample is tested, OTA aptamers conjugating with OTA on their active sites result in the detachment of OTA aptamers from their cDNA. Without the nanostructure amplifier, this NA biosensor significantly decreased its electrochemical performance and switched to a “signal-off” state, thus lowering the electrical signals. This biosensor can detect OTA concentration as low as 8.9 fg/mL in aqueous samples (
Figure 7(Ab,Ac)). A surface-enhanced Raman scattering aptasensor has also been assembled for OTA measurement based on the Ti
3C
2O
2 MXene. The Raman signal was enhanced by plasmonic OTA aptamers modified with Au-Ag Janus nanocomposites [
88]. In the absence of OTA, the OTA aptamer/Au–Ag conjugates bind to the MXene surface by hydrogen bond and chelation interaction between the phosphate group of ssDNA and Ti ions, providing an amplified Raman signal. However, in the presence of OTA, the OTA aptamer/Au–Ag conjugates dissociate from the MXene surface since the active sites of ssDNA are blocked by OTA. The Raman signal thus was attenuated to MXene internal signal without Au–Ag Janus nanocomposites. Consequently, an ultralow LOD of 1.28 pM was achieved and was used to test red wine samples for OTA. The presence of OTA was also detected by a DNAzyme-based biosensor [
89]. The Ti
3C
2 MXene-TiO
2 nanocomposites were doped with Au@PtAg nanoparticles that were a part of the platform for facilitating the photogenerated electron transfer, supporting the ferrocene-labeled duplex DNA probe. With DNAzyme cascade amplification, this photoelectrochemical DNAzyme-based biosensor can rapidly measure the concentration of OTA from 5 fg/mL to 10 ng/mL with a LOD of 1.73 fg/mL. Therefore, this device might be more effective for on-site detection of OTA.
Figure 7. (
A) (
a) Diagram of the assembly process of electrochemical aptasensor for OTA analysis. (
b) Voltammetric response of aptasensor in the presence of different OTA concentrations. (
c) Relationship between the electrochemical response and OTA concentration. Inset. The linear relationship between the response and OTA concentration. Reproduced with permission from Ref. [
87]. Copyright 2022, Elsevier. (
B) (
a) The construction of CRISPR/Cas12a-based fluorescent biosensor for AFB1 determination. (
b) Measurement of AFB1 level in 12 peanut samples by the constructed fluorescent biosensor. (
c) Pictures of 12 peanut samples tested positive (red) and negative (green). Reproduced from Ref. [
92].
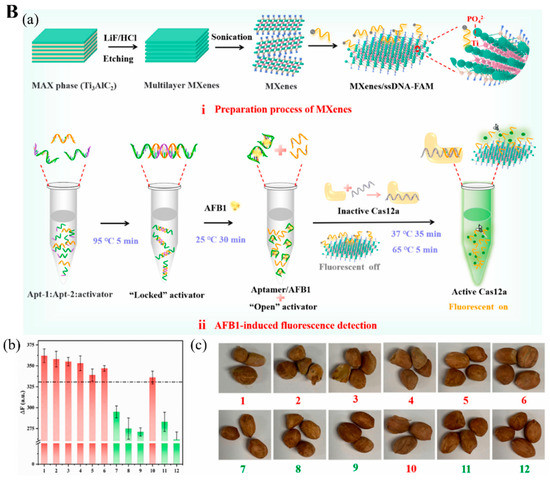
The clustered regularly interspaced short palindromic repeats and specific proteins (CRISPR-Cas) technique was also used for detecting aflatoxin B1 (AFB1) and deoxynivalenol (DON) [
92,
94]. AFB1 is a secondary toxic metabolite released from
Aspergillus flavus and
A. parasiticus, and
A. nominus, frequently found in various foods, including peanuts, grains, and animal feed [
114]. Wu et al. [
92] explored a MXene-based fluorescence biosensor incorporated with the CRISPER-Cas12 technique for AFB1 measurement. When AFB1 is present in the sample, it conjugates with one ssDNA, a locked activator in the form of helical double-strand aptamer opens and releases an aptamer to activate the inactive Cas12a protein linked with guide RNA (crRNA) and thus cleaves the quenched fluorophore-modified ssDNA immobilized on the MXene as small fragments (
Figure 7(Ba)). These fragments then leave the MXene and are dispersed into the solution, recovering the fluorescence signal to the aqueous environment. Their tests in 12 peanut samples for AFB1 were highly accurate (
Figure 7(Bb,Bc)).
Lin et al. [
94] designed a luminescent aptasensor for the detection of DON using an aptamer to activate Cas12 protein trans-cleavage activity that facilitated the luminophore released from the MXene-Au platform but was suppressed in the presence of DON, which finally produced an on/off signal.
Other mycotoxins, such as gliotoxin containing di- or polysulfide bridges, can induce cytotoxicity in humans and animals [
115]. A tetrahedral DNA nanostructure (TDN) was grown on the surface of Ti
3C
2 MXene with one extended capture aptamer in a stand-up posture from a vertex. Combined with the merit of signal enhancers, this MXene-based TDN biosensor can measure gliotoxin in human serum samples as low as 5 pM [
96].
3. Antibiotics Detection
The presence of antibiotics and antibiotic-based medicines is also among the public concerns regarding food safety. The use of antibiotics and related drugs in agri-food systems is often necessary to curb bacterial invasion. Nevertheless, excessive or improper usage of antibiotics leads to food and feed sources laced with antibiotic residues. Consumption of such food and feed products can not only cause several adverse side effects in humans and animals, but also enhance antibacterial resistance [
116,
117].
Chloramphenicol (CAP) is one of the common antibiotics banned from food production due to its potential for bone marrow aplasia, gray-baby syndrome, etc., but it still probably exists in foods of animal origin [
118]. Yang et al. [
100] developed an electrochemical aptasensor using CAP aptamer immobilized on the MXene surface for the detection of CAP in honey as low as 0.03 pM. In another effort, Jiang et al. [
101] employed ZnO quantum dots/nitrogen (N)-doped MXene as a sensing framework that supported aptamers to interact with CAP specifically. Their electrochemiluminescence aptasensor could detect CAP in water and milk with a LOD of 0.019 ng/mL and a linear range from 0.1 to 100 ng/mL.
Another important antibiotic family is aminoglycosides, which are natural or semisynthetic actinomycetes derivatives, including streptomycin (STR), kanamycin, neomycin, tobramycin, etc. [
119]. You et al. [
102] devised a photoelectrochemical “on-off-on” aptasensor for STR measurement with STR aptamer-modified Bi
4VO
8Br/Ti
3C
2 nanostructure. The ability of the Bi
4VO
8Br/Ti
3C
2 nanostructure to generate photogenerated signals is efficiently inhibited by the STR aptamer, resulting in an “on-off” signal framework. When STR is present, the STR aptamer–STR conjugate lowers the inhibition ability of STR. Hence, the photoelectrochemical signal from Bi
4VO
8Br/Ti
3C
2 is again generated. This “on-off-on” aptasensing scheme was able to measure STR concentration in honey in the range of 1 to 1000 nM. In another electrochemical aptasensor, polyethyleneimine (PEI) was used in a metal–organic framework (PEI-UIO-66-NH
3) to coat the polyaniline-Ti
3C
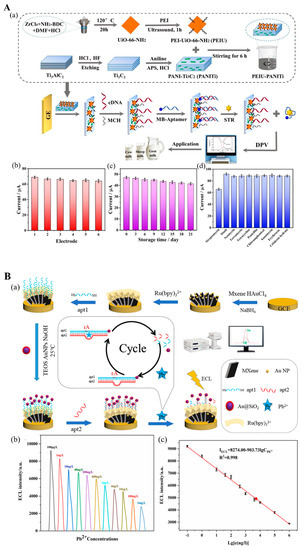
2 MXene surface (PANI-MXene), which was used to modify a glassy electrode (
Figure 8(Aa)) [
103]. After incubating with cDNA, the electrode was ready to immobilize the STR aptamer-methylene blue (MB) structure, which is released from the electrode in the presence of STR. This aptasensor successfully detected 0.01 to 200 nM STR in milk (
Figure 8(Ab–Ad)).
Figure 8. (
A) (
a) Illustration of STR electrochemical aptasensor construction. (
b) Electrochemical response for six replications of STR detection. (
c) Aptasensor stability after storing for 21 days. (
d) Response of electrochemical signal for STR and interference evaluation. Reproduced with permission from Ref. [
103]. Copyright 2022, Elsevier. (
B) (
a) Illustration of electrochemiluminescent (ECL) DNAzyme-based biosensor assembly for Pb
2+ detection. (
b) Responses of ECL of assembled DNAzyme-based biosensor in the presence of various Pb
2+ concentrations. (
c) The linear range of ECL biosensors for Pb
2+ measurement. Reproduced with permission from Ref. [
107].
MXene-based NA biosensors have also been developed for the detection of enrofloxacin and ciprofloxacin [
104,
105]. Enrofloxacin and ciprofloxacin are synthetic fluoroquinolone antibiotics and are used in veterinary drugs. Jiang et al. [
104] fabricated an electrochemiluminescence aptasensor based on O-terminated Ti
3C
2 MXene doped with AgBr nanocrystals. The LOD of this sensor for enrofloxacin in pond water was 5.97
[Math Processing Error] 10
−13 mol/L. The aptamer/Ti
3C
2-Bi
4VO
8Br-TiO
2 nano-construction was used for photoelectrochemical detection of ciprofloxacin, which provides the effectiveness of health implications to milk samples [
105].
4. Other Targets
MXene-based NA biosensors have also been developed for the detection of other target analytes in the agri-food systems, such as the presence of heavy metals. Metal ions are essential to life. However, overconsumption of heavy metal-containing food and feed products causes health issues, e.g., cancer, renal damage, nervous system disruption, etc. Mercury (Hg
2+) and lead (Pb
2+) poisoning are responsible for several diseases and even death due to the ingestion of contaminated food or drinks [
1]. In an electrochemical biosensor designed by Liu et al. [
106], Ti
3C
2T
x MXene/Nafion was modified by GR5 DNAzyme that can cleave the ribo-adenine (rA) site of the DNA substrate in the presence of Pb
2+. Here, Nafion with high viscosity can function as an adhesive not only for Ti
3C
2T
x MXene-GR5 DNAzyme binding but also for Ti
3C
2T
x MXene immobilization over the sensing substrate. After DNA substrate cleavage, the absorptivity of MXene is largely improved, facilitating the adoption of GR5 DNAzyme and ion intercalation, thus increasing its electrochemical performance. This DNAzyme-based biosensor can detect the Pb
2+ effectively and selectively for food safety and environment monitoring. Zhai et al.[
107] employed an electrochemiluminescent biosensor for Pb
2+ in four water samples. They used MXene@Au loaded with tris(2,2-bipyridyl) ruthenium (II) (Ru(bpy)
32+) as a transducer platform to support the aptazyme and its rA-containing DNA substrate labeled with Au@SiO
2 luminophore (
Figure 8(Ba)). Pb
2+ ions activated the endonuclease behavior of the aptazyme that cut the rA sites of the DNA substrate, leading to the luminophore-tailed sequence of DNA substrate releasing into the solution, thus reducing the electrochemiluminescence signal generated from the biosensor. Based on this, they achieved an LOD of 0.059 ng/L over the detection rage of 0.1 to 10
6 ng/L for Pb
2+ (
Figure 8(Bb,Bc)).
A DNAzyme-based fluorometric biosensor was proposed by Lu et al. [
110] for the detection of Hg
2+. Principally, Ti
3C
2 MXene nanosheets adsorbed the fluorophore-labeled ssDNA by metal chelation interaction and hydrogen bond but quenching the fluorescence effect of a fluorophore. The introduction of H
2+ ions initiated the hybridization reaction between the exposed sequence of hairpin DNA and free primers (hairpin-Hg
2+-primer) that were further digested by exonuclease III. Then, the hairpin-Hg
2+-primer structure was broken down into several small ssDNA sequences and nickers that hybridized with fluorophore-tailed ssDNA, thus facilitating the fluorophore-tailed ssDNA separated from the MXene surface to generate a fluorescent signal. This sensor was capable of detecting Hg
2+ in water as low as 42.5 pM.
Other deleterious contaminants in the agri-food systems include pesticide residues. As an organophosphorus insecticide and acaricide, isocarbophos (ICP) can induce several acute toxicities and are carcinogenic to humans. Zhi et al. [
111] developed a Ti
3C
2 MXene@Au sol support ICP aptamer probe using the catalytic activity of the sol to reduce mandelic acid-chloroauric acid (MA-HAuCl
4) for a dual-mode measurement of the ICP concentration in farmland wastewater with high stability, reproducibility, and specificity.
This entry is adapted from the peer-reviewed paper 10.3390/bios12110982