5. Discussion
Mineral extraction brings with it the generation of waste, such as tailings and slags. It is important to find new ways to treat these wastes and extract as many valuable species as possible. The variability of elements in each sample makes leaching a complex process. Hydrochloric acid leaching is an alternative when it is desired to extract more than one element at a time.
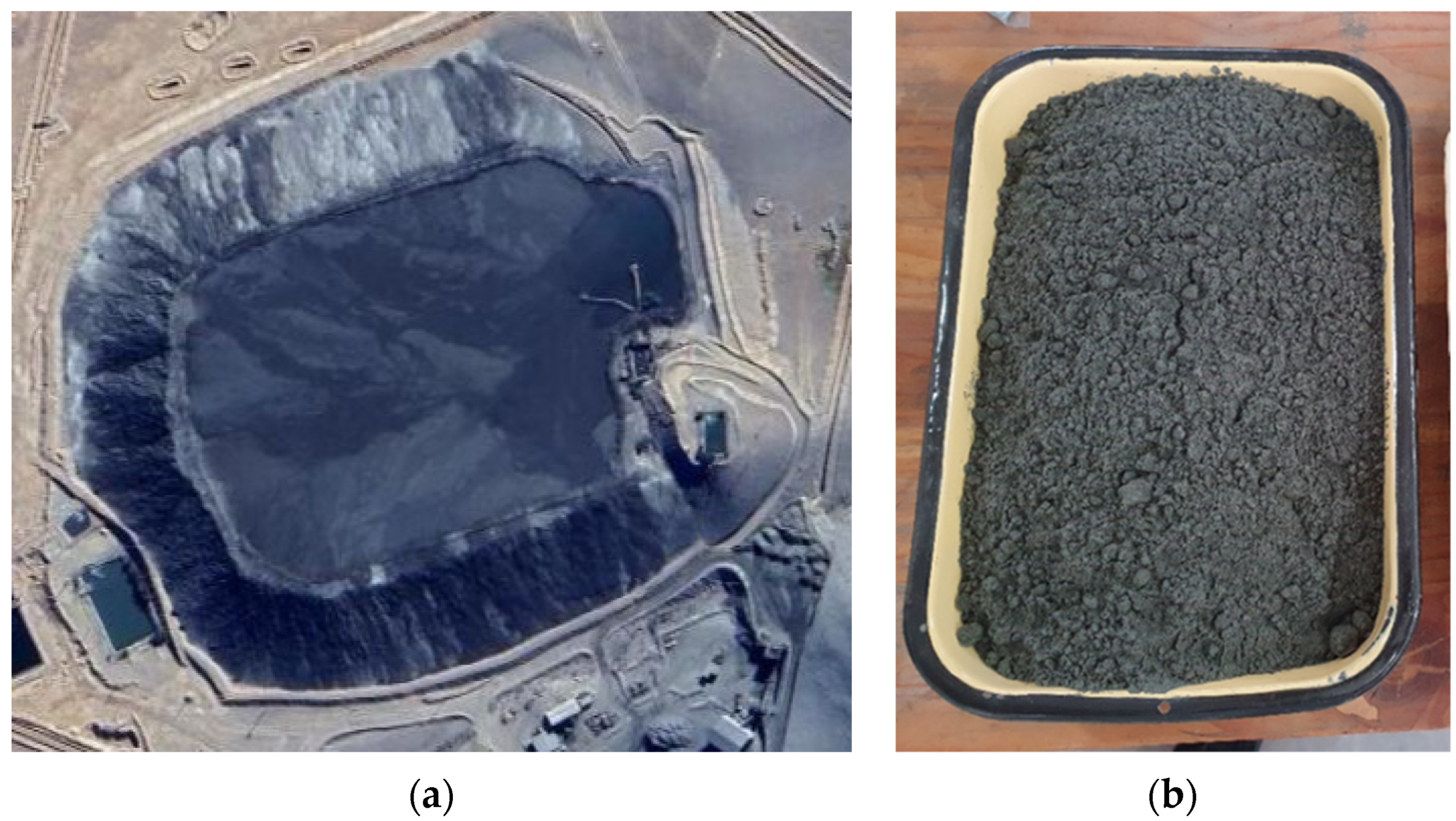
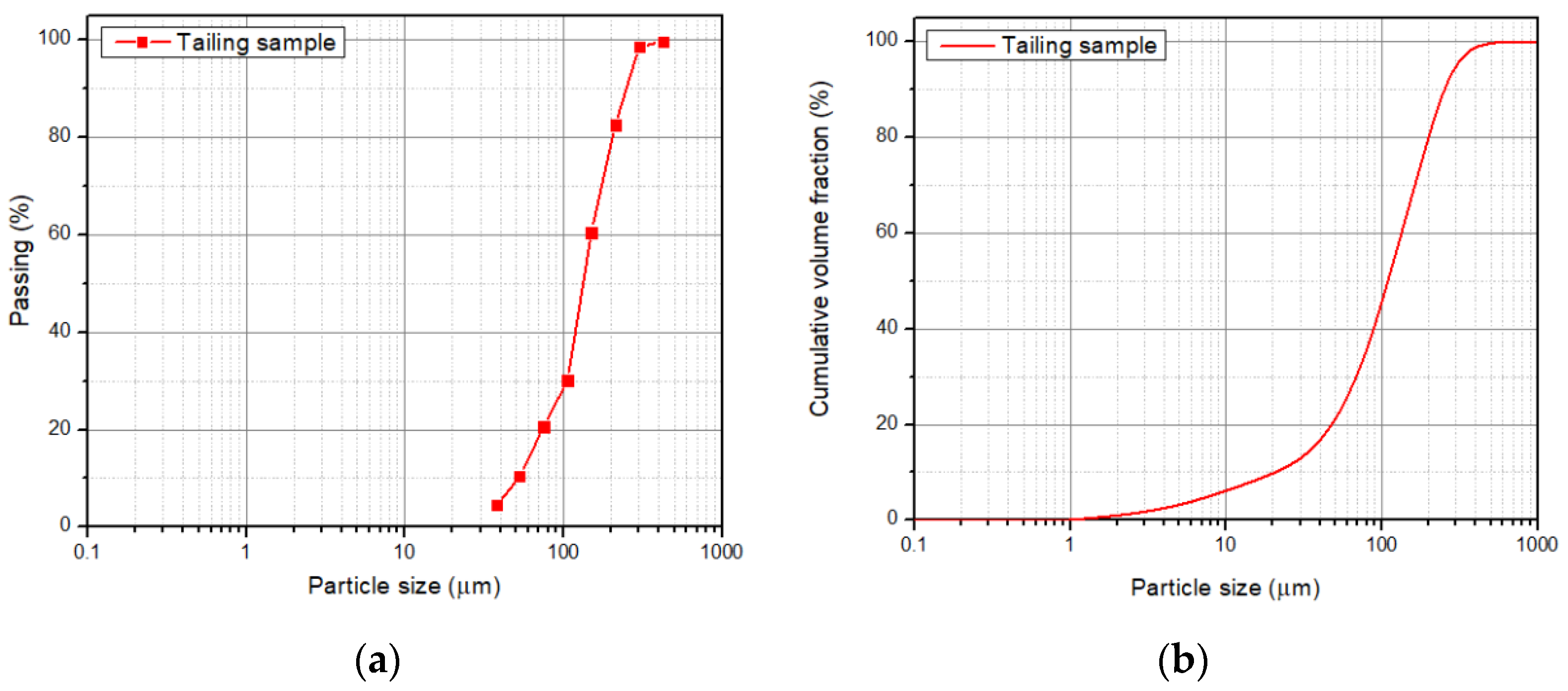
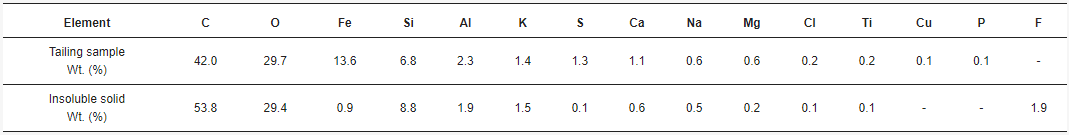
Based on the analysis of the tailing sample from northern Chile, it can be observed that an advantage of it is the high concentration of iron, having a total iron grade of 19%. Size distribution analyses by sieving and Laser Diffraction show that P80 is in the order of 220 µm (
Figure 3). This fine size distribution eases the leaching process given that the iron particles are released
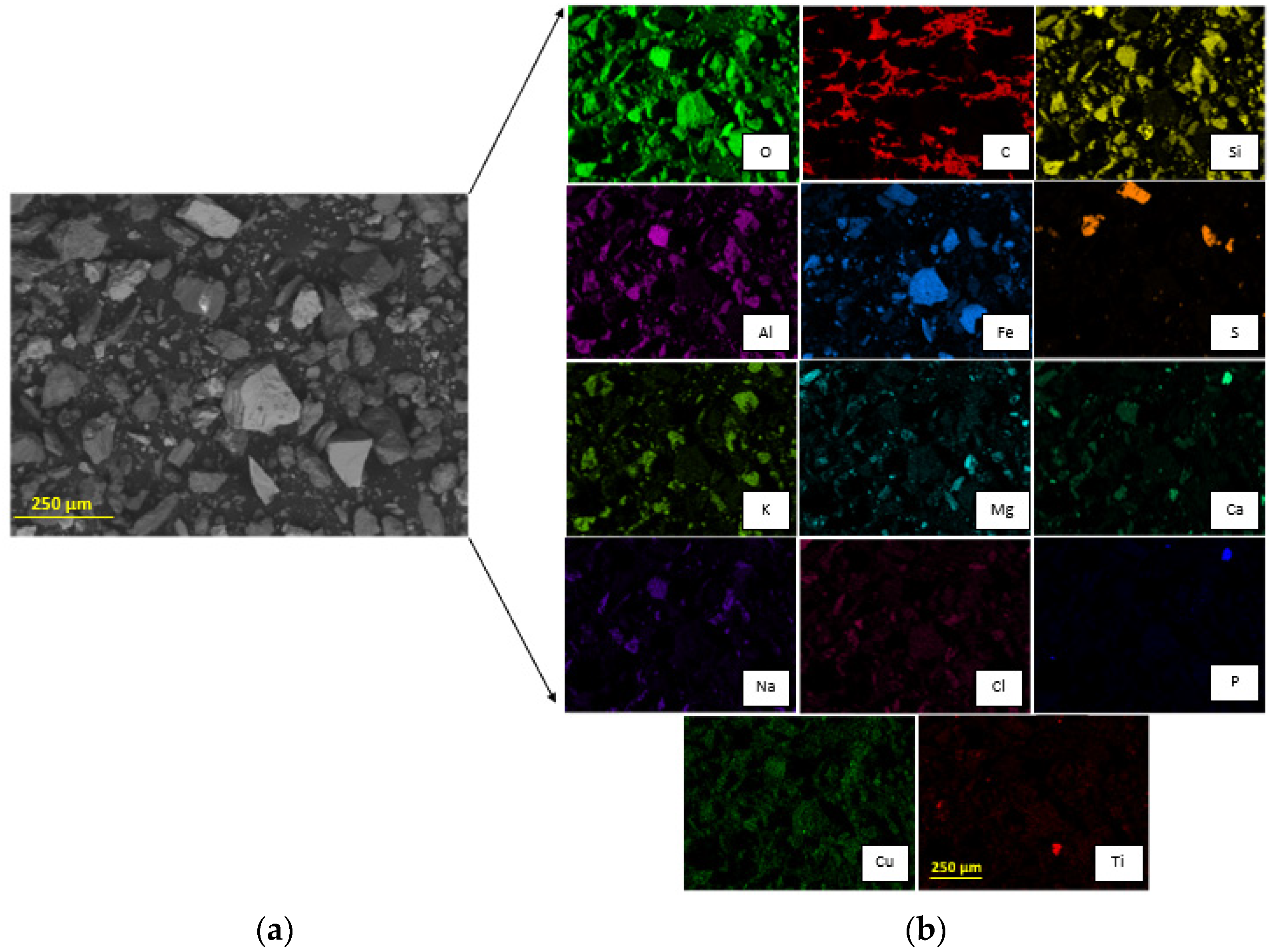
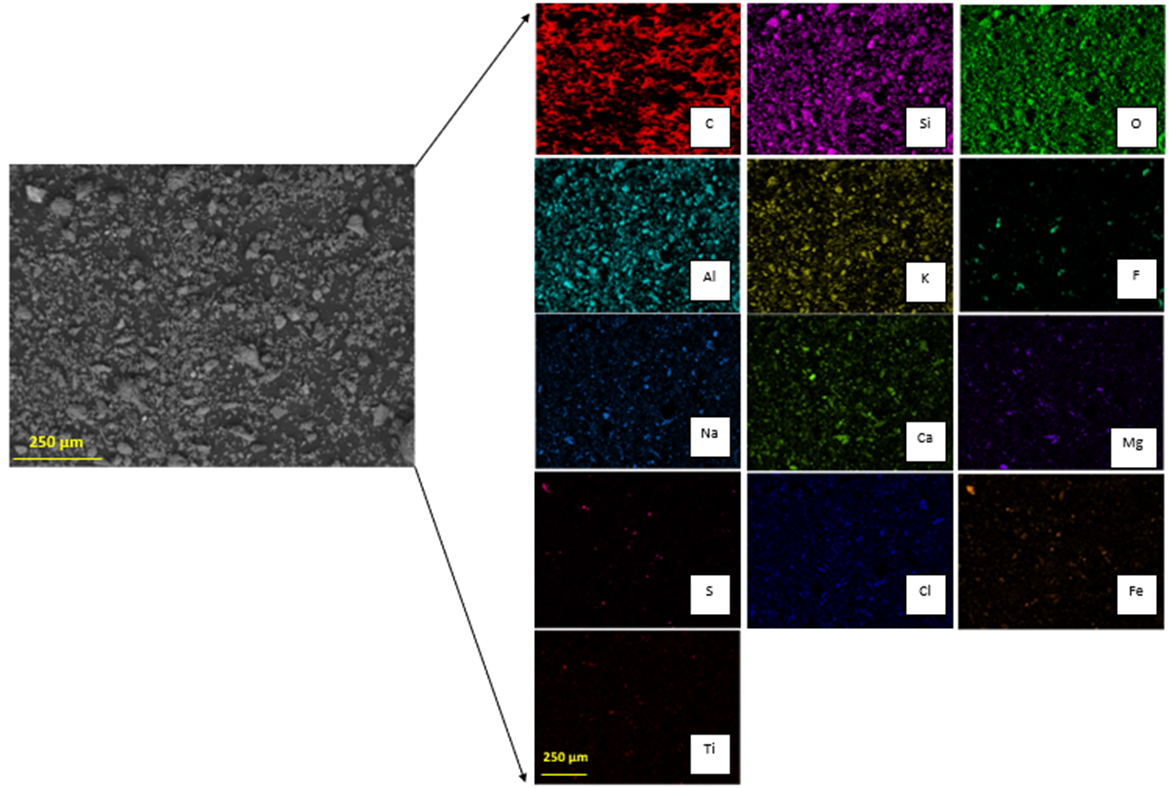
[19]. From SEM analysis it is possible to observe a dispersion of particles in the same size order (
Figure 4). The EDX characterization (
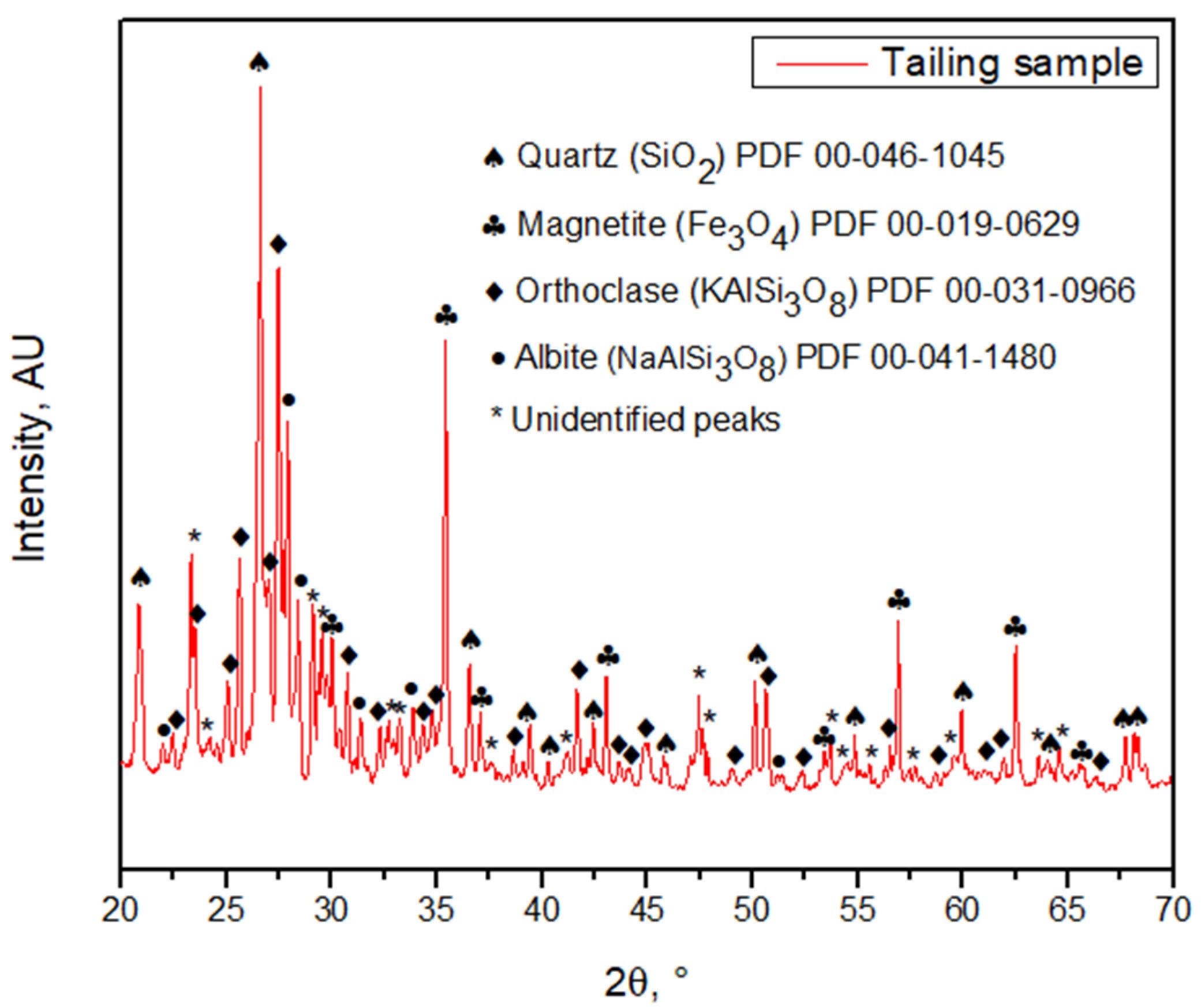
Figure 4) allows us to observe that iron is present in two forms: iron oxide and iron sulfide. Copper can be observed as an oxide, and aluminum, calcium, and magnesium as silicates. Accordingly, SEM-EDX analysis indicates that copper is homogeneously distributed over the sample surface, unlike titanium, which can be observed as discrete particles. Iron is associated with oxygen and sulfur, showing that there could be species such as magnetite, hematite, or pyrite in the sample. Magnesium, calcium, sodium, chlorine, and copper, are homogeneously distributed in the sample and seem to be linked with oxygen; in addition, sodium seems to be related to aluminum. Discrete particles associated with phosphorus and calcium are observed. Titanium is seen as a bright particle and is also distributed through the image, being possibly related to oxygen. Note that carbon is not part of the sample, but is part of the graphite sheet support. The tailing sample characterization shows an association between oxygen and most elements, such as potassium, aluminum, and silicon, probably forming orthoclase, and possibly forming hematite or magnetite with iron. This is confirmed by the XRD analysis since the main minerals are quartz, magnetite, orthoclase, and albite (
Figure 5). Other species may be present, but in concentrations less than 3% by volume, which is the detection limit of the XRD equipment. Having only Kα radiation from Cu and no other tubes, it is challenging to eliminate the fluorescence of Fe. Nevertheless, this composition correlates with the studies conducted on different tailings from Chile
[6][7].
Acid leaching is an efficient conventional technology applied to iron extraction, as it has been proven to have good tailing dissolution
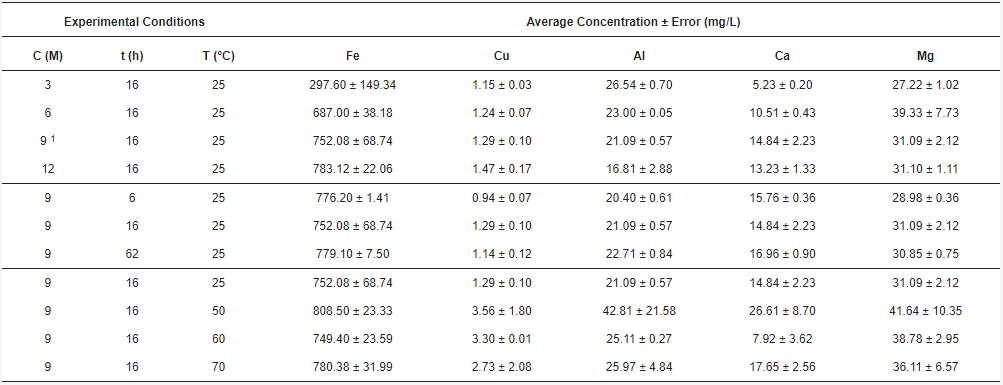
[5][20]. In this research, the concentration of some metallic elements was monitored from the acid solution and tabulated (
Table 1). The average concentration of Fe was plotted as a function of acid concentration, time, and temperature, to analyze its behavior, as shown in
Figure 8. It is seen in
Figure 8a that the iron concentration increases as the acid concentration grows. This is in agreement with previous studies carried out with hydrochloric acid
[13]. Therefore, according to the proposed method (
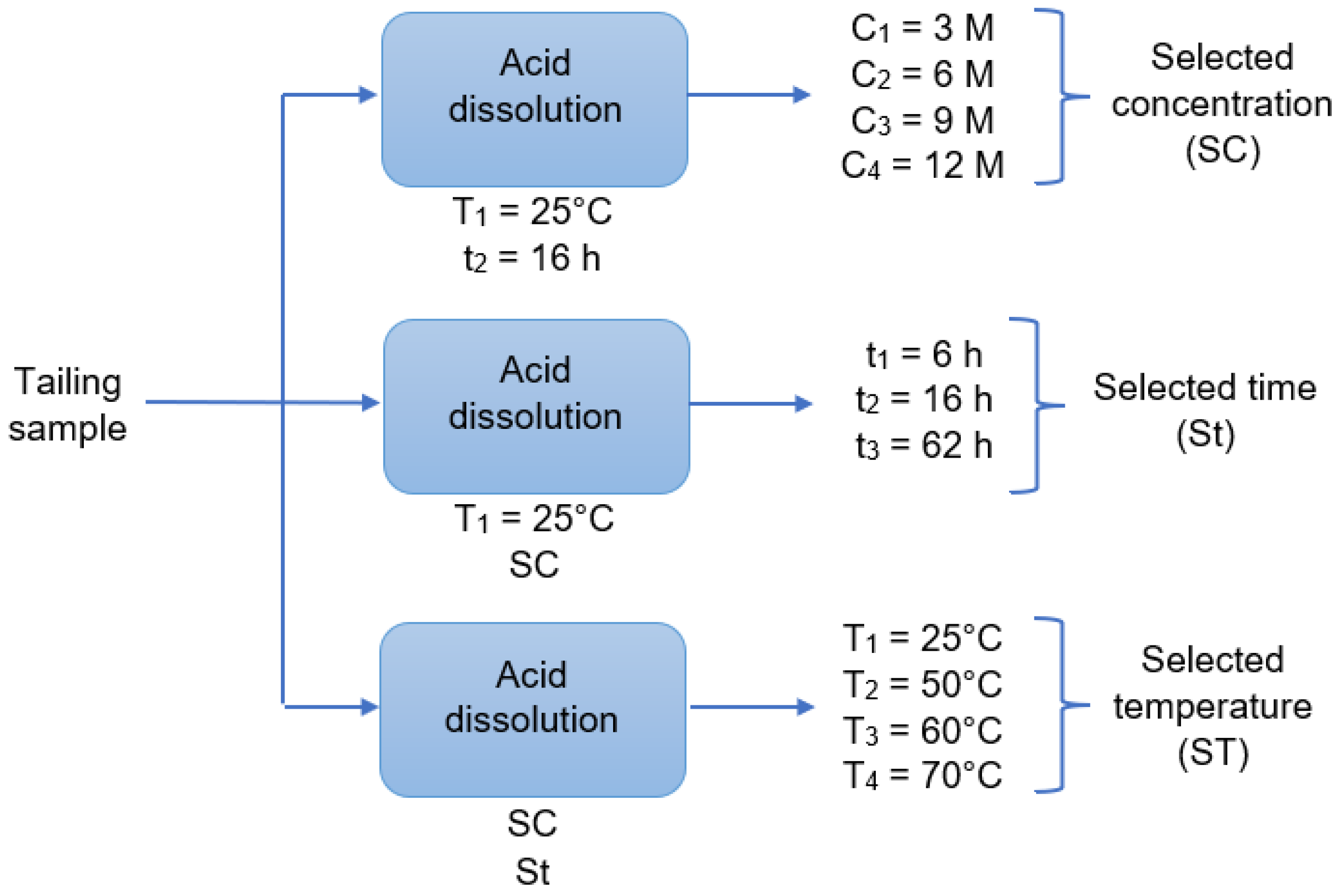
Figure 2), after using 3, 6, 9, and 12 M of HCl concentration at 16 h and 25 °C, the 9 M concentration was selected. This is because the concentrations of the extracted elements are balanced with the increase in acid, e.g., at 9 M, the iron concentration is high and has a variation that can equal the concentration obtained at 12 M (
Figure 8a). Concerning acid dissolution, results showed that the tailing contained an important amount of iron, in the range of 752 mg/L when the conditions are 9 M, 16 h, and 25 °C (
Table 1). Afterwards, it is seen in
Table 1 that time does not influence the iron concentration, as the behavior is practically constant, remaining in the order of 749.40 and 808.50 mg/L (
Figure 8b). This can be attributed to the fact that iron leaching is usually achieved within a few hours
[21]. According to the proposed method, the time was selected in 16 h as it achieves the best extraction of copper and also magnesium. Following the last section of
Table 1, the temperature remains between 749.40 and 808.50 mg/L, which is practically constant as seen in
Figure 8c. This can be related to the complementary effect between acid concentration and temperature
[22]. Thus, since the HCl concentration selected was high, the temperature required for the metal extraction is low. Therefore, the most convenient is to use room temperature at 25 °C.
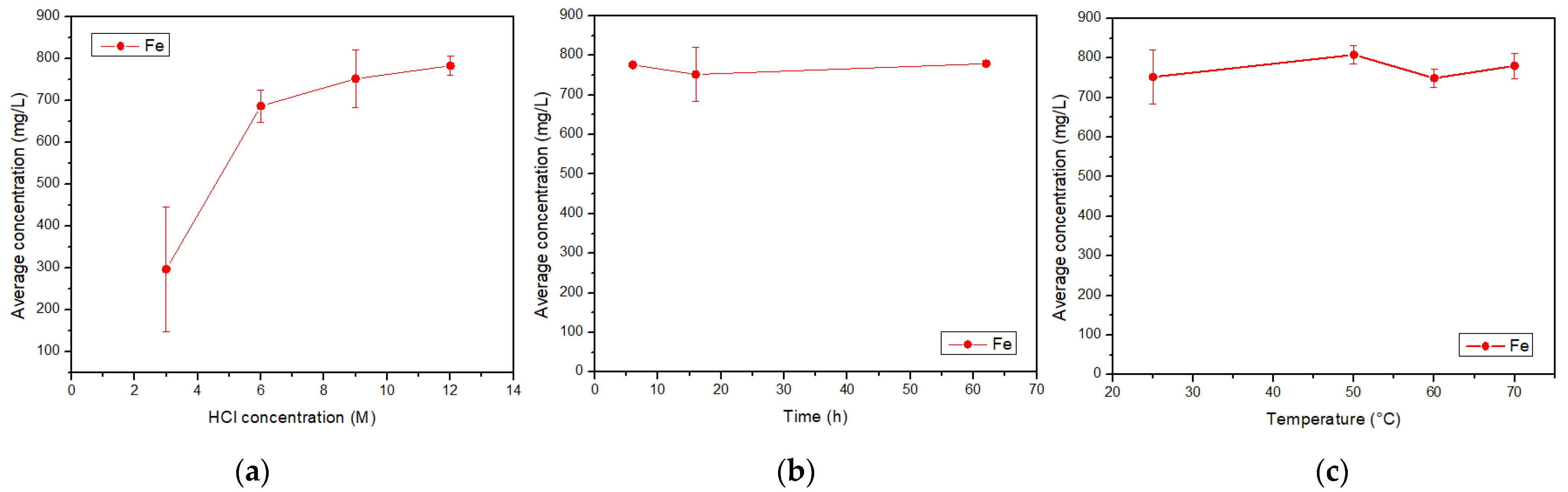
Figure 8. Average concentration of Fe as a function of (a) HCl concentration, (b) time and (c) temperature.
The importance of the other metallic elements contributed to analyze the complexity of the leaching. As seen in Table 1, copper is not affected by acid concentration as it remains between 1.15 and 1.47 mg/L, and aluminum decreases with acid concentration from 26.54 to 16.81 mg/L. Regarding calcium and magnesium, they reach their highest yields at 9 and 6 M, respectively. Thus, the chosen elements are Cu, Al, Ca, and Mg in the same acid solution. Their average concentrations are plotted in a radar chart (Figure 9) showing the variation in front of the HCl concentration, time, and temperature of the reaction. It can be seen that iron has a significant concentration compared to the other elements, e.g., copper had to be converted from mg/L to cg/L to be seen in the radar chart. For copper, the concentration yields at 16 h, while aluminum remains in the range of 20.40 and 22.71 mg/L whether the time is 6, 16, or 62 h. Calcium remains in the range of 14.84 to 16.96 mg/L, and magnesium is between 28.98 and 31.09 yielding at 16 h. Additionally, for copper, aluminum, calcium, and magnesium, the highest concentrations are obtained at 50 °C, whether they do not show a clear tendency. In Figure 9a, it is possible to see that Cu, Ca and Mg tend to have higher concentrations between 6, 9, and 12 M, with 3 M not being enough to extract these elements. On the contrary, Al tends to be higher at lower acid concentrations. With Figure 9b it is seen that a time between 16 and 62 h is suitable for a successful extraction of these elements, being enough with 16 h. Figure 9c shows that 50 °C is enough to obtain high concentrations of Cu, Al, Ca, and Mg, as higher temperatures do not increase the concentration of these elements. However, it was experimentally determined that a room temperature of 25 °C is enough to extract iron (Figure 8c) and to maintain a more stable and efficient energy system.
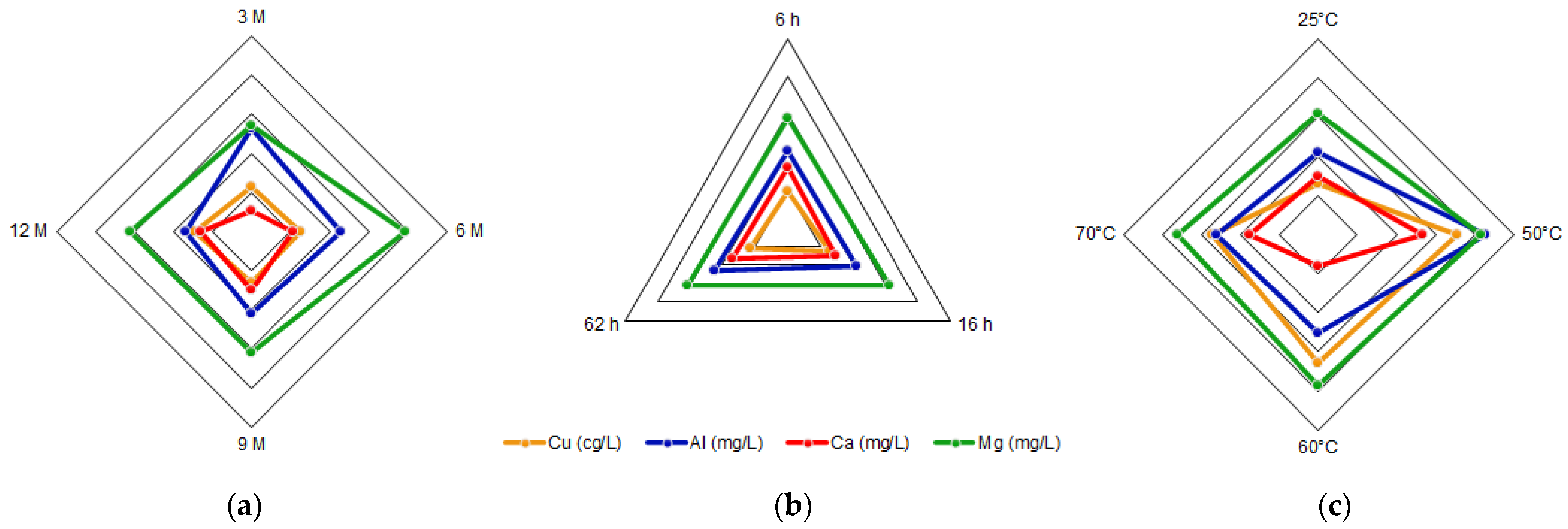
Figure 9. Radar chart of Cu, Al, Ca and Mg average concentrations as a function of (a) HCl concentration, (b) time and (c) temperature.
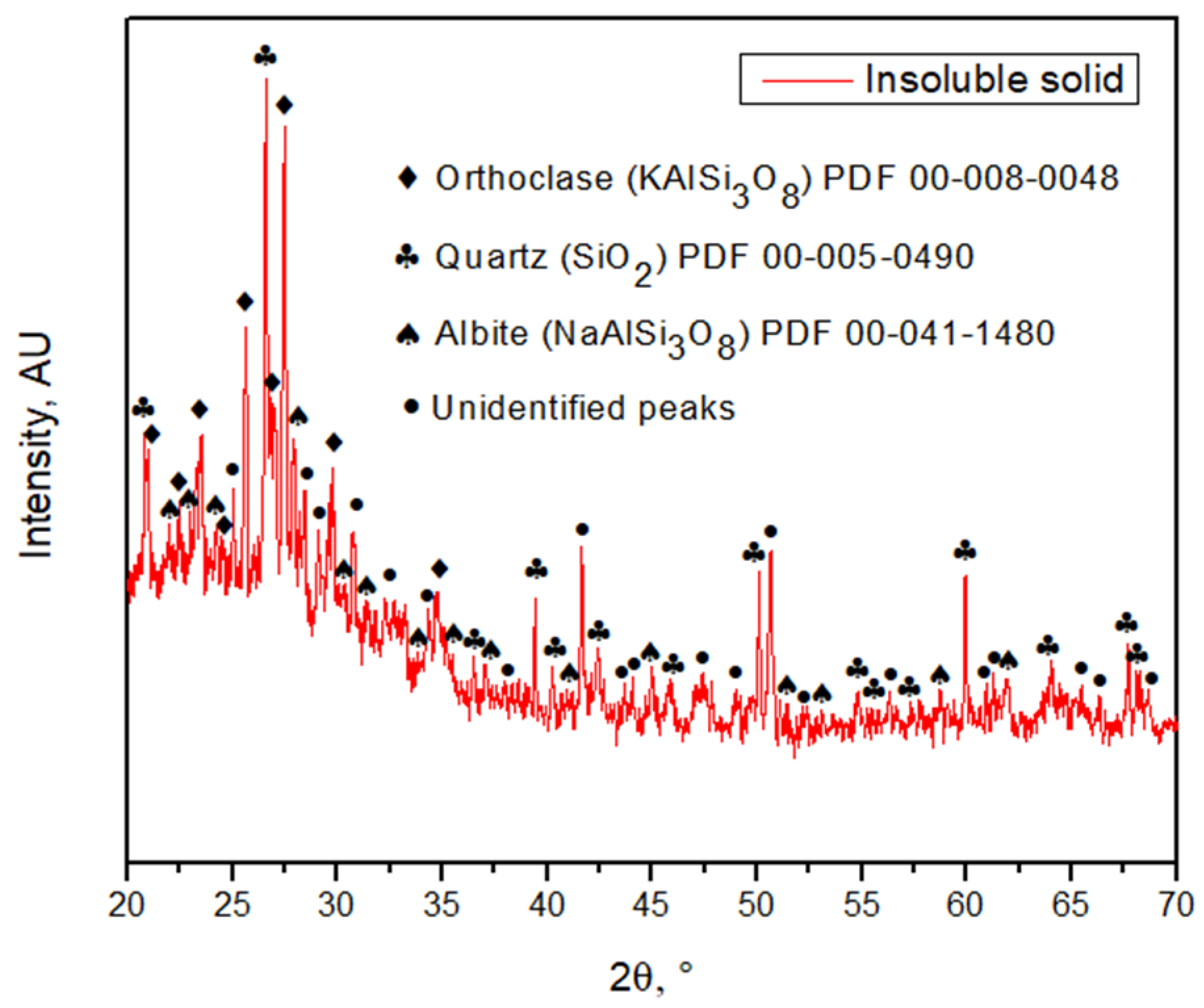
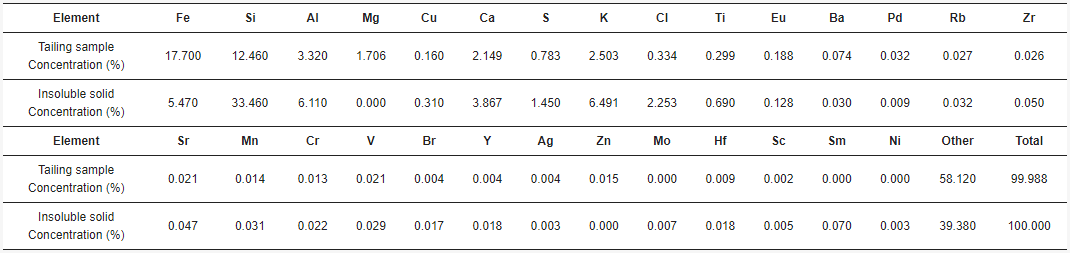
After acid dissolution, the main elements in the products are silica, oxygen, iron, potassium, and aluminum. Iron and aluminum are expected to be in the acid solution, and silica along with potassium is expected to be in the insoluble solid. Regarding the insoluble solid obtained after the leaching process (Figure 6), it was determined that it contains silicates that should be in a purer form, given that the presence of iron is low and it is associated with sulfur. Meaning that silicates associated with potassium, calcium, and sodium persist after leaching with acid (Figure 6), showing that the valuable metals contained in the tailing will be present in the acid solution after the leaching process, and the minerals related to silica will be in the insoluble solid retained in the filter. Table 2 shows that oxygen and iron are the most present elements in the tailing sample. As previously discussed, carbon is not part of the sample, but is part of the graphite sheet support, so it is not part of the tailing. Regarding the insoluble solid sample, it can be seen (Table 2) that a minimal amount of iron stays with the solid retained in the filter, so iron is mainly in the acid solution. Almost all the silicon is present in the solid. Since copper is not detected in the solid, it must have been dissolved in the acid solution. Part of the aluminum is in the solid forming orthoclase together with silicon. Small amounts of calcium and magnesium stay in the solid. It is noticed that silicon, oxygen, aluminum, potassium, and sodium are part of silica, orthoclase, and albite, as shown in the XRD (Figure 7). On the other hand, calcium and magnesium, sodium with chlorine, and iron with sulfur, seem to be related. A minimum amount of titanium is seen as particles possibly related to oxygen. There is also the presence of fluorine as particles on the sample that might be related to fluorosilicates. Table 3 shows the chemical composition of the tailing sample and in the insoluble solid with more detail. Elements such as Cr, V, and Eu, were not detected by the previous techniques. Regarding the main element analyzed, the insoluble solid contains only 5.47% Fe in comparison with the tailing, which contains 17.70% Fe, meaning that most of the iron was dissolved.
Given that the elements of interest were extracted from the copper tailing, future research contemplates the treatment of the acid solution to form a product containing valuable species
[23].
6. Conclusions
The tailings of northern Chile contain an important amount of iron and other valuable species, such as copper and aluminum, which can be extracted through leaching with hydrochloric acid. The most relevant condition in this process is the acid concentration, due to its effect on the extraction of metals. Conditions can be selected according to the element that wants to be obtained, e.g., if a high iron extraction is wanted, a high acid concentration should be applied, while on the contrary, if a high extraction of aluminum is wanted, a low acid concentration should be used. The variables time and temperature do not show a relevant influence on the iron concentration, so it can be considered that it presents a constant behavior. Within the ranges studied, the conditions of 9 M acid concentration, 16 h reaction time, and a temperature of 25 °C were selected as the optimum leaching parameters. This process differs from traditional leaching by using hydrochloric acid instead of sulfuric acid; in addition, the raw material is a residue from a metallurgical process instead of mined ore. This proves that valuable elements such as iron, copper, and aluminum, along with calcium and magnesium, can be extracted from copper tailings for further valorization. The silicates obtained in the insoluble portion show low iron concentrations and could be used in other applications such as additives in the construction industry.