Tuberculosis (TB) is a contagious bacterial illness known to humankind since ancient times. The causal microorganism of TB is Mycobacterium tuberculosis (Mtb). Lung or pulmonary TB is the most common form, but Mtb also affects other body parts. TB does not spare any age group and is omnipresent worldwide. Most TB patients remain asymptomatic (latent TB) and non-contagious. However, approximately 10% of latent TB cases may advance to active TB (active or symptomatic TB). Some usual symptoms of active TB comprise continuing chronic cough, hemoptysis, night sweating, and weight loss. Active TB is associated with a high mortality rate if left untreated. The 2021 TB report of the World Health Organization (WHO) states that TB is one of the top 10 reasons for global deaths, about one-quarter of the global population is affected by Mtb, and the global burden of TB is expected to increase due to the COVID-19 pandemic.
- tuberculosis
- drug-resistance
- Mmpl3
- SQ109
- clinical studies
- patent
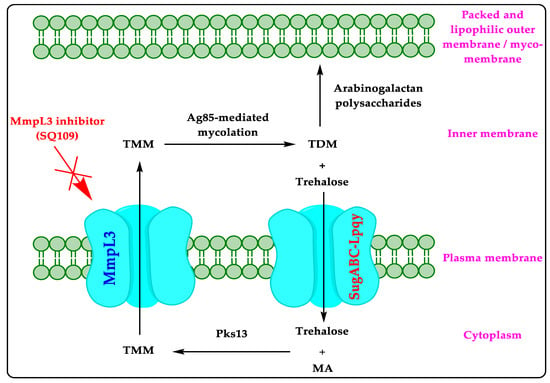
1. Mycobacterial Membrane Protein Large 3 (MmpL3)
2. Literature on MmpL3 Inhibitors
| Ref. No. | Year | Summary of the Review Article |
|---|---|---|
| [14] | 2020 | Reviews MmpL3 (physiological role, structure, and properties) and ligands/inhibitors of MmpL3 (AU1235, ICA38, SQ109, rimonabant, SPIRO, and NITD-349). |
| [16] | 2021 | Describes MmpL3 as a drug target, different chemical classes of MmpL3 inhibitors (derivatives of indole carboxamide, pyrrole/pyrazole, quinoline/quinolone, adamantane, benzimidazole, acetamide, and spiro-compound) and different ligands of MmpL3 like ICA38 (PDB ID: 6AJJ), rimonabant (PDB ID: 6AJI), SQ109 (PDB ID: 6AJG), and U1235 (PDB ID: 6AJH). |
| [20] | 2022 | Reviews indole derivatives as MmpL3 inhibitors (NITD-349, indolamide, adamantanol analogs, and indole-2-carboxamides) and inhibitors of other anti-TB drug targets (InhA, DprE1, KasA, chorismate mutase, DNA replication, DNA gyrase, dihydrofolate reductase). |
| [21] | 2022 | Describes MmpL3 as a promiscuous drug target and also spotlights the MmpL3 inhibitors of different chemical classes (adamantyl derivatives, piperidinol derivatives, and benzimidazole derivatives). It also highlights other anti-TB drug targets (TrmD, Ag85C, GyrB, and ClpC1). |
| [22] | 2021 | Briefly explains clinical study data (NCT01785186) of SQ109 (an MmpL3 inhibitor) and comments on the improved anti-TB activity of SQ109 with MDR regimens. |
| [23] | 2020 | Talks about MmpL3, the preclinical/clinical development of MmpL3 inhibitors (BM212, THPP, SQ109, Spiro, NITD-349, NITD-304, AU1235, C215, and HC2091), and different chemical classes of MmpL3 inhibitors (derivatives of indole, benzimidazole, benzothiazole, piperidine, 4-Thiophen-2-yloxane-4-carboxamide, benzofuran, quinoline/quinolone, naphthalene, acetamide, and pyrrole). |
| [24] | 2020 | Explores the current development of MmpL3 inhibitors (ethylenediamine derivatives, carboxamide derivatives, benzothiazole amides, adamantyl ureas, pyrroles and pyrazoles, benzimidazoles, spiropiperidines, and piperidinol), along with their structure-activity relationship (SAR) and challenges in developing them. It also provides the chemical structure of many MmpL3 inhibitors (AU1235, CRS400393, BM212, THPP, spiropiperidine, TBL-140, ICA38, HC2091, BM533, BM635, rimonabant, C215, PIPD1, NITD-349, NITD-304, and SQ109,). |
| [25] | 2020 | Surveys the new targets for TB, including MmpL3 and the chemistry of MmpL3 inhibitors (design and structural features) in clinical/preclinical trials. |
| [26] | 2020 | Identified lead compounds from PubChem database targeting MmpL3 and other anti-TB drug targets by high-throughput screening. |
| [27] | 2019 | Underlines the chemical structures and designs of MmpL3 inhibitors. |
| [28] | 2018 | Highlights the target validation, discovery, hit-optimization, and SAR of MmpL3 inhibitors of different chemical classes (ethylenediamine, adamantyl ureas, phenyl pyrroles, benzimidazoles, indole carboxamides, and spiropiperidines). |
| [29] | 2014 | Discloses MmpL3 as a validated target for developing anti-TB medications. It also discloses SQ109 and BM212 as MmpL3 inhibitors. |
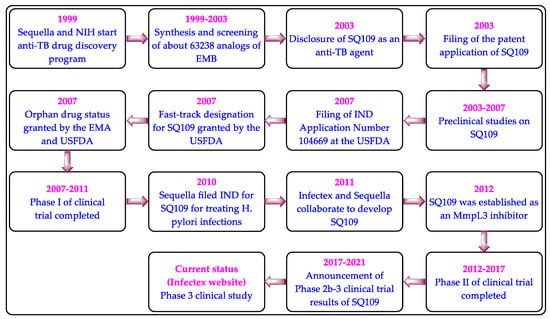
3. Clinical Studies on MmpL3 Inhibitors
4. SQ109
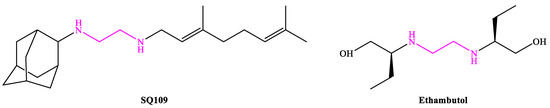
4.1. Mechanism of Action
4.2. Preclinical Studies
| The Anti-TB Activity of SQ109 | The Anti-TB Activity of SQ109 Combinations in Mice | ||||
|---|---|---|---|---|---|
| Susceptibility Profile | Assay | MIC (μg/mL) | Drug Regimen | Log10 CFU in Lung | Log Decrease |
| H37Rv (pan-susceptible) | BACTEC | ≤0.2 | Two weeks | ||
| H37Rv (pan-susceptible) | Alamar | ≤0.39 | Untreated | 6.16 ± 0.02 | - |
| Erdman (pan-susceptible) | Alamar | ≤0.39 | INH + RIF + EMB | 4.64 ± 0.23 | 1.52 |
| EMB-resistant | Alamar | 0.78 | INH + RIF + SQ109 | 4.46 ± 0.12 | 1.70 |
| INH-resistant | Alamar | 0.78 | Four weeks | ||
| RIF-resistant | Alamar | ≤0.39 | Untreated | 6.42 ± 0.76 | - |
| XDR plus EMB-resistant | Microbroth | 0.20 | INH + RIF + EMB | 3.86 ± 0.14 | 2.56 |
| INH + RIF + SQ109 | 3.26 ± 0.12 | 3.16 | |||
4.3. Clinical Studies on SQ109
| Title (Allocation; Intervention Model; Masking; Purpose) | Intervention and Active Comparator (AC) | NCT Number (Status; Phase; Number Enrolled; Results; Outcome Measures) |
Sponsor/Collaborator (Location; Study Start Date (SSD); Study Completion Date (SCD); Last Update Date (LUD)) |
|---|---|---|---|
| Pharmacokinetics and early bactericidal activity (EBA) of SQ109 in adult subjects with pulmonary TB (Randomized; Parallel assignment; None (Open-label); Treatment of TB) |
SQ109 monotherapy (75 mg, 150 mg, and 300 mg tablet daily) or a combination of RIF with SQ109 (RIF standard dose + 150 mg or 300 mg of SQ109) for 14 days; AC: RIF capsule (150 mg) | NCT01218217 (Completed; 2; 90; Not available; EBA of SQ109 monotherapy and combination therapy of SQ109 with RIF) |
Michael Hoelscher and Sequella, Inc. (South Africa; November 2010: May 2012; 14 January 2013) |
| Evaluation of SQ109 plus PPI in urea breath test-positive volunteers (Not mentioned; Single group assignment; None (Open-label); Treatment of H. pylori infection) |
SQ109 (300 mg) daily for two weeks; AC: Not mentioned | NCT01252108 (Withdrawn due to lack of funding: 2: 0: Not available; safety and efficacy of SQ109 against H. pylori infection in adult patients) |
Sequella, Inc. (Not mentioned; March 2012; August 2015; 17 November 2015) |
| Evaluation of SQ109, high-dose RIF, and moxifloxacin in adults with smear-positive pulmonary TB in a MAMS design (Randomized: Single group assignment: None (Open-label): Treatment of TB) |
Combinations of SQ109 (300 mg) with RIF (10 to 35 mg/kg), INH (75 mg), PZA (400 mg) and pyridoxine (25 mg); AC: Combination of INH, RIF, PZA, and EMB |
NCT01785186 (Completed: 2: 365; Available; Two negative sputum cultures utilizing liquid media) |
Michael Hoelscher and Sequella, Inc. (South Africa; April 2013; March 2015; 20 September 2017) |
| Escalating single-dose safety, tolerability, and pharmacokinetics of SQ109 in healthy volunteers (Randomized; Single group assignment; Quadruple (participant, care provider, investigator, outcomes assessor); Treatment of TB) |
A single oral dose of SQ109 (10 mg, 20 mg, 50 mg, 100 mg, 200 mg, 300 mg, and the combination of fatty food with 300 mg of SQ109); AC: Placebo | NCT01585636 (Completed; 1; 62; Not available; Safety and pharmacokinetics of single dose of SQ109 for seven days) |
Sequella, Inc. and Quintiles, Inc. (United States; September 2006; February 2007; 19 August 2013) |
| Dose escalation study of SQ109 in healthy adult volunteers (Randomized; Parallel assignment; Double (participant, investigator); Treatment of MDR-TB) |
SQ109 (75 mg and 150 mg) daily for 14 days and SQ109 (150 mg) daily on days 1–5, 9, and 14; AC: Placebo | NCT00866190 (Completed; 1; 10; Not available; Safety and tolerability evaluation of SQ109) |
National Institute of Allergy and Infectious Diseases (NIAID) (United States; April 2009; November 2009; 6 November 2011) |
| Phase IC study of safety and PK of SQ109 300 mg daily (Randomized; Parallel assignment; Triple (participant, investigator, outcomes assessor); Treatment of TB) |
A single dose of SQ109 (300 mg) daily for two weeks; AC: Placebo | NCT01358162 (Completed: 1: 10: Not available: Safety and tolerability evaluation of SQ109) |
NIAID (United States; November 2010; April 2011; 14 May 2013) |
| Effects of SQ109 on QTc interval in healthy subjects (Randomized; Crossover assignment; None (Open-label); Treatment of TB) |
Oral SQ109 (300 mg or 450 mg daily) for seven days; AC: Placebo | NCT01874314 (Withdrawn due to undisclosed reason; 1; 0; Not available; Effect of SQ109 on QTc interval) |
NIAID (United States; Not available; December 2015; 24 March 2014) |
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10112793
